Author: Aditya Gilda
Mentor: Dr. Ziv Ben-Zion
Normanhurst Boys High School
Abstract
Recently, more and more strains of bacteria have become resistant to modern antibiotics and have raised the issue of treating these resistant strains. However, developing new antibiotics and vaccines to combat these new variants is both expensive and unable to solve the underlying problem. A more novel method of treatment involving bacteriophages proposes a solution that could be both less costly and solve the underlying problem of evolving antibiotic resistance. Bacteriophages were discovered in 1915, but research about possible treatments using the phages were abandoned in favor of the cheaper alternative presented by the discovery of antibiotics. The discovery of antibiotics eventually resulted in commercialisation and overproduction, the primary reason for the unintentioned production of antibiotic resistant strains of bacteria. The aim of this review is to evaluate the effectiveness of bacteriophages against antibiotic resistant pathogens compared to more conventional treatment methods and their practical advantages. This will include an evaluation of the effectiveness of clinical trials against MDROs – in particular- Acinetobatcer Baumannii, an ESKAPE pathogen placed under the highest priority by the WHO in its ‘list of drug-resistant bacteria most threatening to human health’. By analyzing the potential side-effects, risk of future resistance and effectiveness, the advantages and disadvantages of phage therapy compared to antibiotics can be contrasted in the hope of further identifying reasons for integrating phage therapy into modern medical practices.
Introduction
As of 2021, an estimated 1.4 million deaths were attributable to pathogens with antimicrobial resistance (Naghavi et al., 2024), a number that will only continue togrow as more and more pathogens evolve to combat the antibiotics meant to kill them. In the last decade, research focusing on bacteriophage vaccines and phage therapy has significantly increased and has presented a potential solution to the problem of antimicrobial resistance (AMR) (Abushaheen et al., 2020).
Bacteriophages (phages for short) are naturally occurring viruses that prey on bacteria. Due to the specificity of their chosen prey, bacteriophage vaccines only infect bacteria and do not harm human cells and our microbiome, in comparison to antibiotics which do not distinguish and kill all bacteria affected. Phages can also be genetically engineered to only target specific bacteria and certain phages are inherently able to provoke an immune response, vital in producing a vaccine that actively involves the immune systems, resulting in long term immunity. Phages are capable of being mass produced via hosts (e.g. E Coli, Staphylococcus aureus, Pseudomonas aeruginosa, etc.)and – through genetic engineering – they can be used to express specific antigens in a stable and reliable way.
Phage therapy involves using bacteriophages to specifically target and kill bacteria and presents several advantages. While antibiotics can have lethal effects against a wide range of bacteria, phages are highly specific and reduce side-effects and danger to beneficial bacteria. Many phages are also lytic – when a bacteria is infected it bursts, releasing clones of the phage that infected it – increasing the concentration of phages at the site leading to higher doses at sites of infection.
Phage vaccines (a type of phage therapy) work by artificially engineering phage to express specific antigens of their surface, meant to stimulate an immune response and alert the immune system to the invading pathogen and its unique antigens. This exposure also serves to create a memory response which provides long term protection against the pathogen.
Here, we aim to discuss the advantages of phage therapy over antibiotics and how they present a viable solution to the growing problem of AMR. This review is based on key papers in the topics of Antimicrobial resistance, Bacteriophage, Phage Therapy, Acinetobacter baumannii, and antibiotics.
Keywords: Antimicrobial resistance, Bacteriophage, Phage Therapy, Acinetobacter baumannii, antibiotics
A. Antimicrobial resistance (AMR) and antibiotics
The exponential number of antibiotics used today are the main factors driving the development of bacterial AMR. By placing such severe and constant selective pressure, the ultimate result is a population of bacteria resistant to modern antibiotics, that evolves faster than the development of new drugs. The constant race to find a better chemical to kill these bacteria is not a feasible option and new methods must be developed to effectively solve this problem. In order to do so, the effects and phenotypic characteristics of AMR must be better understood. Through the example of Carbapenem-resistant Acinotebacter baumannii (A.baumannii), we can see how a pathogen has adapted to the presence of antibiotics and the results of this selective pressure (see section A2).
A.1. Causes and characteristics of bacteria with AMR
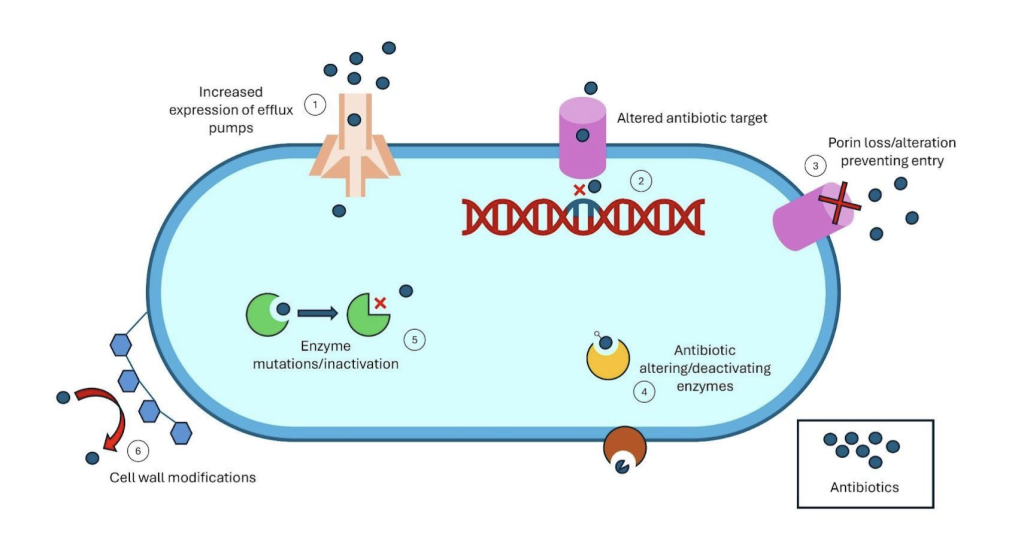
AMR is a pressing global health issue, posing a significant threat to modern medicine. The misuse and overuse of antimicrobials in humans, animals, and agriculture have accelerated the emergence and spread of drug-resistant pathogens. Different classes of antibiotics attack and kill bacteria in different ways and work by interfering with vital microbial functions such as mechanisms of the cell wall and protein synthesis, often being used in combination (Abushaheen et al., 2020). In recent times, multidrug resistant organisms (MDROs) have captured greater global attention with some bacteria such as A.baumannii having varying levels of resistance to almost all modern antibiotics (CDC, 2024). Microbes may develop resistance via either genetic mutations or horizontal gene transfer from other bacteria (Gómez-Piña et al., 2024).
The primary treatment for A. baumannii are Carbapenems (e.g., imipenem, meropenem, doripenem) (A type of beta-lactam antibiotic highly effective againstmost bacteria) . However, A.baumanni has evolved several mechanisms to developresistance to carbapenems (and other key drugs, via the production of beta-lactamases (an antimicrobial-inactivating enzymes), increased number of efflux pumps and alterations in membrane permeability and antibiotic target sites (Shi et al., 2024).
A.2. Key mechanisms of AMR in bacteria treated by antibiotics
Acinetobacter baumannii, along with other MDROs, has developed a variety of strategies to resist antibacterial agents. It achieves this resistance in three main ways. First, by limiting access to its targets through reduced membrane permeability and the overexpression of efflux pumps, particularly those from the resistance-nodulation-division (RND) family. These efflux pumps, common in gram-negative bacteria like A. baumannii, can lower the intracellular levels of many standard antibiotics. Second, it modifies its target sites through genetic changes that make antibiotics ineffective sharing those beneficial mutations via methods such as horizontal gene transfer which has been noticed in high percentages in samples of A.baumannii. Third, it can directly inactivate antibiotics through the production of beta-lactamase, carbapenems and other enzymes which inhibit the performance of antibiotics (Kyriakidis et al., 2021).
A. baumannii is also capable of forming biofilms—structured communities of bacteria encased in a protective extracellular matrix. These biofilms act as both a physical and chemical barrier against antibiotics, often reducing their effectiveness by over 80%. The resistance associated with biofilms is further supported by genes like ‘bap’ and ‘ompA’ , which help enhance the formation and stability of these biofilms (Roy et al., 2022).
B. Medical uses of bacteriophages
To combat the growing problem of bacteria with AMR, Bacteriophage vaccines and phage therapy have been proposed as a novel way of targeting these pathogens, potentially solving the root problem in a cost effective and safe way. Bacteriophages, as mentioned before, are naturally occurring predators of bacteria but can be genetically engineered and selected (in a process called phage screening) in order to provoke, involve and ‘teach’ the immune system to recognise and act against pathogens.
Another method – homologous recombination – uses infected bacteria and foreign DNA to alter the genome of the phage which has infected the bacteria. The result of this is the release of the altered phages upon bacterial lysis allowing scientists to design phages with a specificity for certain strains of bacteria.
Both methods can also be used in combination with antibiotics to more effectively combat bacteria, resulting in a more foolproof treatment method with less chance of failure. These treatments present many future possibilities and open the way for further research and development as well as a deeper understanding of the role and significance of phages.
B.1 Development, production and physiology of bacteriophages
Bacteriophages are viruses that specifically target bacteria and archaea and consist of genetic material wrapped up in protective protein structures known as capsids. One interesting technique that emerged in 1985 is called phage display. This method allows scientists to express peptides or proteins on the surface of the phage by merging the DNA that codes for the desired polypeptide with the genes for phage coat proteins. This way, the polypeptide is showcased outside the phage’s capsid (Aghebati-Maleki et al., 2016). Once these phage particles are displaying the polypeptides, they go through screening processes to find those with the right binding properties, like a strong affinity or specificity for certain pathogens. Large collections of these phage-displayed peptides are then tested against specific targets, such as bacterial receptors or toxins, which helps in selecting the phages that interact with these targets (Petrenko & Vodyanoy, 2003). Through multiple rounds of selection and amplification—a process known as biopanning—phages that show the most promising characteristics are separated and cultured. These can then be utilized as precise tools to combat multidrug-resistant pathogens by specifically targeting and neutralizing certain bacterial strains, like Acinetobacter baumannii.
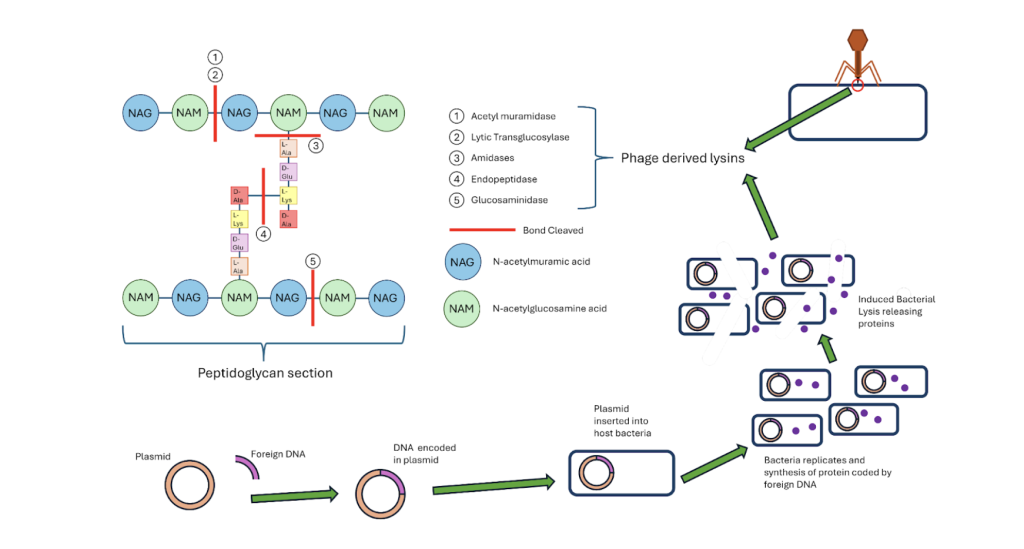
Another common method used is homologous recombination, used to change the genome of the phages allowing the alterations of receptors. This method involves the integration of a piece of foreign DNA into a plasmid which is inserted into a host bacteria. The same bacteria is infected with a phage, causing the production of phages within the bacteria with the inserted DNA integrated into the phage’s genome. Upon lysis, the phages are released and those carrying the added DNA arescreened and separated, allowing scientists to design phages which specifically target the pathogen needed potentially leading to greater treatment success. (Pines et al., 2015)
B.2 How phages can be used to combat bacteria with AMR
Bacteriophages offer a promising defence against A. baumannii as well as other MDROs. Here, we will focus on A. baumannii as a key example. Lysins, protein-based enzymes in bacteriophages, are able to cleave covalent bonds of the peptidoglycan layer of bacterial cell walls, leading to rapid destruction of the bacterial cell wall without toxicity to mammalian cells (Borysowski et al., 2011). These lysins are found in the tail of bacteriophages (allowing the phage to penetrate) and can be manufactured through the insertion of a plasmid coding the desired lysin into a host bacteria which can manufacture the lysin, releasing it upon death. These enzymes are grouped in 5 main categories each severing a specific bond in the peptidoglycan matrix of the bacterial cell wall (see Figure 1) (Golban et al., 2025). This action is particularly effective against A. baumannii, as its biofilm matrix, which shields the bacteria from antibiotics, can be broken down by phage-derived lysins and depolymerases (enzymes located primarily in the tail spike of the phage. This allows the penetration of the biofilm, exposing the bacteria to further attack (Grygiel et al., 2024). In addition, bacteriophages can infect A. baumannii through their tail fiber proteins, which recognize and bind to specific receptors on the bacterial surface to allow precise infection and lysis (Liu et al., 2025) The bacteriophage’s limited host range prevents unintended damage to beneficial bacteria and surrounding cells, minimising unwanted destruction, while their capacity for co-evolution with resistance mechanisms in bacteria renders them an active agent against antibiotic-resistant disease. With the addition of antibiotics, phages become even more effective, providing a multi-pronged approach to block and destroy A. baumannii (Teymouri et al., 2024).
C. Synergy between antibiotics and phage therapy
Alone, phages present a new and formidable method of treatment against MDRO’s and have opened up new pathways in the way we treat bacterial infections. However, the target pathogen may develop resistance mechanisms to render the phages less effective – similar to how many of the antibiotics we have relied on until now have a decreased effectiveness against pathogens they were formerly deadly against. Phages are capable of evolving alongside the pathogens they are made to infect, which makes an effective “weapon”. Nevertheless, several studies have suggested a synergistic relationship between antibiotics and bacteriophages. For example, one study conducted on methicillin-sensitive S. aureus found that, when subjected to both antibiotics known to work against a non-resistant strain of the bacteria and the PYOSa lytic phage, the strain developed resistance against the phage. This exposed the bacteria to the antibiotics, against which the mutations developed were ineffective (Tran et al., 2024). This presents a method of treatment with a potential success rate much higher than either phage therapy or antibiotic treatment alone, reducing the risks of reinfection and development of resistance.
Several cases of therapeutic synergy have been observed between antibiotics and phages. One such study noted the effect of sub-lethal doses of beta-lactam antibiotics on a bacterial population infected with phages. The authors observed that the sub-lethal doses of aztreonam and cefixime encouraged high numbers of phages released upon bacterial death, compared to the same strain of bacteria exposed to phages without added antibiotics (Comeau et al., 2007). Other researchers also noted increased susceptibility to antibiotics upon exposure to phages. For example, a strain of A. baumannii, AB900 displayed mutations linked to capsule loss in an effort to evade the phage øFG02, which binds to the bacterial capsule. While this shielded AB900 from the phage, it left very little defence against beta-lactam antibiotics and other phages, which were then able to attack unhindered by the bacterial capsule (Altamirano et al., 2022).
The same study used the AB900 strain of A. baumannii to evaluate the effectiveness of combination therapy in mice. This was done in three groups in comparison to a control group: (1) a phage-antibiotic combination treatment, (2) a phage-only treatment group, and (3) an antibiotic-only treatment group. The results showed that the combination of antibiotics and phage therapy was better than each agent individually. This highlights the effectiveness and potential of combination therapy even against serious infections caused by MDROs. (Altamirano et al., 2022)
On a cellular level, combination therapy displays such a high success rate due to a higher elimination of resistance pathways. By using two or more methods to attack the pathogen, a mutation that might make the pathogen resistant to one drug, may render it helpless against another. This concept has already been applied in antibiotic therapy and further enhanced by adding the constantly evolving and adapting phages. Biofilms, a significant barrier for antibiotics and a main resistance mechanism for pathogens (e.g., as A. baumannii and MRSA), show significant ratesof decline when exposed to a combination of antibiotics and phages. One study showed a significant reduction (i.e., from 50,586 colony-forming-units of MRSA to 5000 units) when exposed to a combination of a bacterium-specific phage Sb-1 and the antibiotic teicoplanin. On the other hand, only smaller reductions were found when using t only phages (recorded number of 30, 788 units) andt only antibiotics (17, 165 units) (Yilmaz et al., 2013).
D. Discussion
Bacteriophage therapy presents a promising way forward and a new outlook in the way we approach bacterial infections. However, this therapy is not without its challenges. Phages apply strong selective pressures on their host bacteria like antibiotics making it possible for bacteria to develop resistance to the phages. Mutations in bacterial receptors limit the rate of phage absorption, also achieved through hiding the receptors behind substances such as slime layers. If the phage is successful in injecting its genetic information into the bacteria, the CRISPR-Cas (REF) resistance mechanism identifies the phage’s DNA to recognize and destroy it in the future. Another effective mechanism is the Restriction-modulation system, an innate immune response which cleaves phage DNA, recognised due to its unmethylated state. As a last resort, the bacteria can also induce its own death, preventing the phage from completing its replication cycle (Kou et al., 2024).
However, by supplementing phage therapy with other measures, treatments can circumvent these resistance mechanisms and still achieve a high success rate. As discussed in section C, a combination of both phages and antibiotics have shown high success rates in multiple studies even against biofilm-forming strains. Studies have also shown how a CRISPR-Cas system represses (in some cases entirely) antibiotic resistance mechanisms in A. baumannii due to the modification of receptors. These receptors enable communication and the sharing of resistance genes between bacteria as well as the removal of efflux pumps, impact on biofilm formation and increased membrane permeability (Wang et al., 2022). To note, phages themselves are capable of evolving and responding to resistance measures in their hosts, part of what makes them such an effective method of treatment, capable of solving issues of AMR in the long-term.
To conclude, phage therapy is a viable and powerful tool against bacteria, especially MDROs such as A. baumannii. While our society still heavily relies on antibiotics, further leaps in our understanding of phages and their interactions, as well as their production, may serve to integrate them into our medical practices.
Acknowledgments:
I would like to thank Dr. Ziv Ben-zion for the insight and guidance offered on the process of constructing and refining this paper.
References
Abushaheen, M. A., Muzaheed, Fatani, A. J., Alosaimi, M., Mansy, W., George, M., Acharya, S., Rathod, S., Divakar, D. D., Jhugroo, C., Vellappally, S., Khan, A. A., Shaik, J., & Jhugroo, P. (2020). Antimicrobial resistance, mechanisms and its clinical significance. Disease-a-Month, 66(6), 100971. https://doi.org/10.1016/j.disamonth.2020.100971
Aghebati-Maleki, L., Bakhshinejad, B., Baradaran, B., Motallebnezhad, M., Aghebati-Maleki, A., Nickho, H., Yousefi, M., & Majidi, J. (2016). Phage display as a promising approach for vaccine development. Journal of Biomedical Science, 23, 66. https://doi.org/10.1186/s12929-016-0285-9
Altamirano, F. L. G., Kostoulias, X., Subedi, D., Korneev, D., Peleg, A. Y., & Barr, J. J. (2022). Phage-antibiotic combination is a superior treatment againstAcinetobacter baumannii in a preclinical study. eBioMedicine, 80. https://doi.org/10.1016/j.ebiom.2022.104045
Borysowski, J., Łobocka, M., Międzybrodzki, R., Weber-Dabrowska, B., & Górski, A. (2011). Potential of Bacteriophages and Their Lysins in the Treatment of MRSA: Current Status and Future Perspectives. BioDrugs, 25(6), 347–355. https://doi.org/10.2165/11595610-000000000-00000
CDC. (2024, April 12). Infection Control. Infection Control.https://www.cdc.gov/infection-control/hcp/mdro-management/background.html
Comeau, A. M., Tétart, F., Trojet, S. N., Prère, M.-F., & Krisch, H. M. (2007). Phage-Antibiotic Synergy (PAS): β-Lactam and Quinolone Antibiotics Stimulate Virulent Phage Growth. PLOS ONE, 2(8), e799. https://doi.org/10.1371/journal.pone.0000799
Golban, M., Charostad, J., Kazemian, H., & Heidari, H. (2025). Phage-Derived Endolysins Against Resistant Staphylococcus spp.: A Review of Features, Antibacterial Activities, and Recent Applications. Infectious Diseases and Therapy, 14(1), 13–57. https://doi.org/10.1007/s40121-024-01069-z
Gómez-Piña, J. J., Luna, V. C., Alfaro, N. L., Reyes, A. G. M., Pérez, C. P., Valdés, N. P., & Acevedo, A. O. (2024). Emerging antibiotic resistance: A modern-day horsemanof the apocalypse. International Journal of Research in Medical Sciences, 12(3), 663–670. https://doi.org/10.18203/2320-6012.ijrms20240504
Grygiel, I., Bajrak, O., Wójcicki, M., Krusiec, K., Jończyk-Matysiak, E., Górski, A.,Majewska, J., & Letkiewicz, S. (2024). Comprehensive Approaches to Combatting Acinetobacter baumannii Biofilms: From Biofilm Structure toPhage-Based Therapies. Antibiotics, 13(11), 1064.
Kou, X., Yang, X., & Zheng, R. (2024). Challenges and opportunities of phage therapy for Klebsiella pneumoniae infections. Applied and Environmental Microbiology, 90(10), e01353-24. https://doi.org/10.1128/aem.01353-24
Kyriakidis, I., Vasileiou, E., Pana, Z. D., & Tragiannidis, A. (2021). Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens (Basel, Switzerland), 10(3), 373. https://doi.org/10.3390/pathogens10030373
Liu, S., Lei, T., Tan, Y., Huang, X., Zhao, W., Zou, H., Su, J., Zeng, J., & Zeng, H. (2025). Discovery, structural characteristics and evolutionary analyses of functional domains in Acinetobacter baumannii phage tail fiber/spike proteins. BMC Microbiology, 25, 73.
Naghavi, M., Vollset, S. E., Ikuta, K. S., Swetschinski, L. R., Gray, A. P., Wool, E. E., Aguilar, G. R., Mestrovic, T., Smith, G., Han, C., Hsu, R. L., Chalek, J., Araki, D. T., Chung, E., Raggi, C., Hayoon, A. G., Weaver, N. D., Lindstedt, P. A., Smith, A. E., … Murray, C. J. L. (2024). Global burden of bacterial antimicrobial resistance 1990–2021: Asystematic analysis with forecasts to 2050. The Lancet, 404(10459), 1199–1226. https://doi.org/10.1016/S0140-6736(24)01867-1
Petrenko, V. A., & Vodyanoy, V. J. (2003). Phage display for detection of biological threat agents. Journal of Microbiological Methods, 53(2), 253–262. https://doi.org/10.1016/S0167-7012(03)00029-0
Pines, G., Freed, E. F., Winkler, J. D., & Gill, R. T. (2015). Bacterial Recombineering: Genome Engineering via Phage-Based Homologous Recombination. ACS Synthetic Biology, 4(11), 1176–1185. https://doi.org/10.1021/acssynbio.5b00009
Roy, S., Chowdhury, G., Mukhopadhyay, A. K., Dutta, S., & Basu, S. (2022). Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection. Frontiers in Medicine, 9. https://doi.org/10.3389/fmed.2022.793615
Shi, J., Cheng, J., Liu, S., Zhu, Y., & Zhu, M. (2024). Acinetobacter baumannii: An evolving and cunning opponent. Frontiers in Microbiology, 15. https://doi.org/10.3389/fmicb.2024.1332108
Teymouri, S., Pourhajibagher, M., & Bahador, A. (2024). A review of the fighting Acinetobacter baumannii on three fronts: Antibiotics, phages, and nanoparticles. Molecular Biology Reports, 51(1), 1044.
Tran, M., Viera, A. J. H., Tran, P. Q., & Mo, C. Y. (2024). Bacteriophage infection drives loss of β-lactam resistance in methicillin-resistant Staphylococcus aureus. eLife, 13. https://doi.org/10.7554/eLife.102743.1
Wang, Y., Yang, J., Sun, X., Li, M., Zhang, P., Zhu, Z., Jiao, H., Guo, T., & Li, G. (2022). CRISPR-Cas in Acinetobacter baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiology Spectrum, 10(4), e00829-22. https://doi.org/10.1128/spectrum.00829-22
Yilmaz, C., Colak, M., Yilmaz, B. C., Ersoz, G., Kutateladze, M., & Gozlugol, M. (2013). Bacteriophage therapy in implant-related infections: An experimental study. The Journal of Bone and Joint Surgery. American Volume, 95(2), 117–125. https://doi.org/10.2106/JBJS.K.01135
About the author

Aditya Gilda
Aditya is currently a Year 10 student at Normanhurst Boys High School. He is really passionate about maths and biology, in particular the fields of immunology and microbiology. Outside of school, Aditya plays the flute and also enjoys art.