Author: Vanessa Georgieva
Mentor: Dr. Ali Rahimpour Jounghani
Hinsdale Central High School
Abstract
Monitoring brain function in athletes is essential for optimizing performance, preventing injury, and ensuring safe recovery. Traditional neuroimaging methods such as PET, MEG, and fMRI provide valuable insights but are limited by cost, immobility, invasiveness, and sensitivity to motion, making them unsuitable for dynamic sports environments. Functional near-infrared spectroscopy (fNIRS) offers a promising alternative: it is portable, non-invasive, motion-tolerant, and capable of monitoring cortical activity in real time. Research demonstrates its utility for studying cognitive load, fatigue, decision-making, and concussion, while its integration with other wearable technologies further enhances its potential in applied sports settings. Nevertheless, challenges such as shallow penetration depth, sparse spatial resolution, and sensitivity to hair and scalp characteristics remain. Ongoing innovations including high-density optode arrays, advanced signal processing, and miniaturized wearable systems are rapidly improving data quality and usability. Together, these developments suggest that portable fNIRS can become a valuable tool in sports neuroscience, bridging laboratory-based methods and real-world athletic performance.
Introduction
In order to ensure athlete safety and avoid serious neurological harm, consistently monitoring brain function is essential. An unavoidable aspect of an athlete’s training involves exposure to both physical and mental stress, along with a constant risk of injury. Certain brain injuries have the potential to deteriorate athletic performance and even long-term brain health. To monitor an athlete’s brain effectively, it is important to study brain activity during actual physical movement.
This requirement limits the use of many conventional neuroimaging techniques including electroencephalography (EEG), functional magnetic resonance imaging (fMRI), and positron emission tomography (PET) as they typically require stillness, are expensive, bulky, difficult to transport, and in some cases involve exposure to radiation. These factors make them unsuitable for use in natural sports environments.
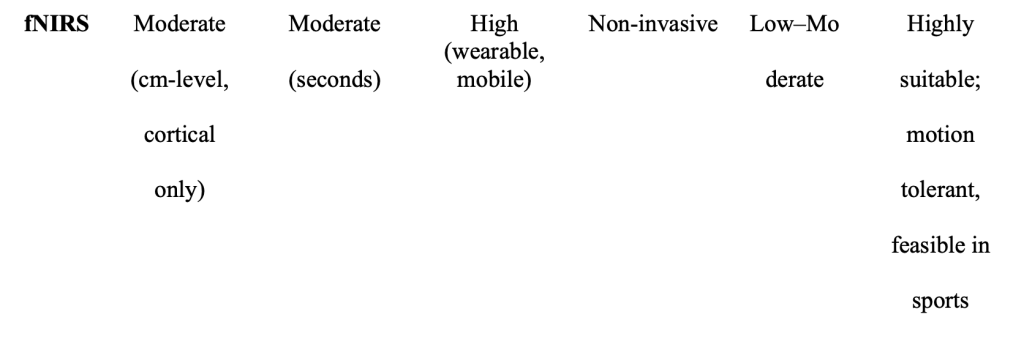
Functional Near-Infared Spectroscopy (fNIRS) is a promising alternative due to its cost effectiveness, portability, and non-invasive nature, which allows real-time monitoring of brain function. Thanks to its flexibility and tolerance to motion, fNIRS has shown promise for studying brain activity during real-world activities such as walking and cycling (Herold, 2018). This makes fNIRS particularly valuable for athletes, whose brain activity can be measured in more ecologically valid settings improving both the accuracy and applicability of the findings. fNIRS holds distinct potential as a tool for studying brain function in athletes during real-world physical activity.
Overview of Neuroimaging Methods
There is a wide range of neuroimaging techniques used to study brain function, each with its own strengths and limitations. Depending on the method’s capabilities, its utility varies across different settings. While many traditional techniques are effective in clinical or laboratory environments, they tend to be less practical for field-based or movement-heavy applications—such as in sports.
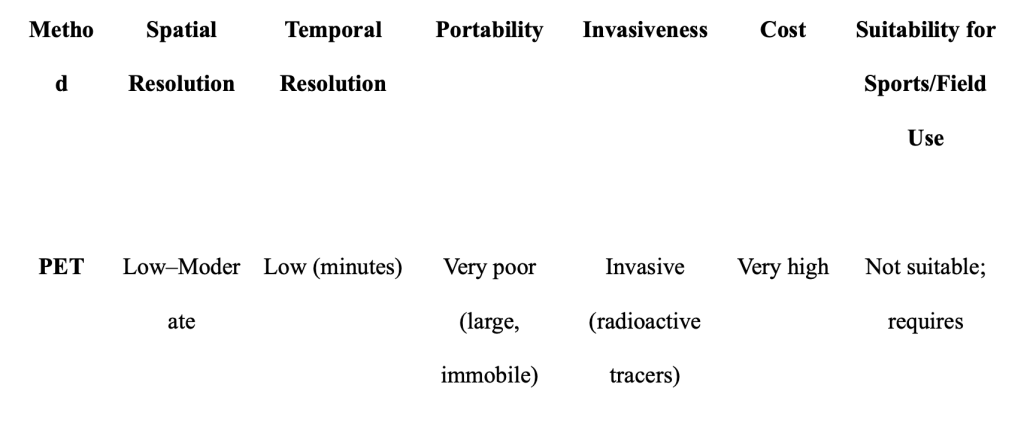
PET is a non-invasive and highly sensitive technique that provides real-time imaging of bodily function. It works by using radionuclides that decay via positron emission. When a positron encounters an electron, both particles are annihilated, releasing two gamma rays in opposite directions. Surrounding detectors capture these rays to pinpoint the origin of the positron within the body. PET is particularly useful in detecting subtle biochemical changes and is widely applied in drug development and clinical fields like oncology, neurology, and cardiology. However, its use of radioactive tracers (which are short-lived and costly), limited anatomical detail unless combined with other imaging, low spatial resolution, and complete lack of portability make it unsuitable for real-world sports applications. Athletes perform rapid, dynamic movements, and PET requires the subject to remain perfectly still. Furthermore, exposing healthy athletes to radiation via tracers poses unnecessary health risks (Cherry, 2001).
MEG is another non-invasive method that measures the brain’s magnetic fields resulting from electrical activity. It offers excellent temporal resolution, making it effective for tracking fast neural events and studying sensory processing, language, attention, and motor control. Despite these advantages, MEG is impractical in athletic settings due to its high cost, large equipment size, and extreme sensitivity to environmental noise. It requires a magnetically shielded room, which is incompatible with data collection in natural, high-movement environments (Baillet, 2017).
fMRI is a widely used, non-invasive technique that measures brain activity indirectly through blood-oxygen-level-dependent (BOLD) signals. It offers high spatial resolution and is valuable for mapping large-scale brain networks involved in tasks, perception, and emotion. However, BOLD does not directly reflect neural activity, and its susceptibility to motion artifacts makes it poorly suited for capturing brain function during physical activity. In athletic contexts, where movement is essential, fMRI’s requirement for stillness and its sensitivity to motion significantly limit its utility (Logothetis, 2008).
Functional Near-Infrared Spectroscopy (fNIRS)
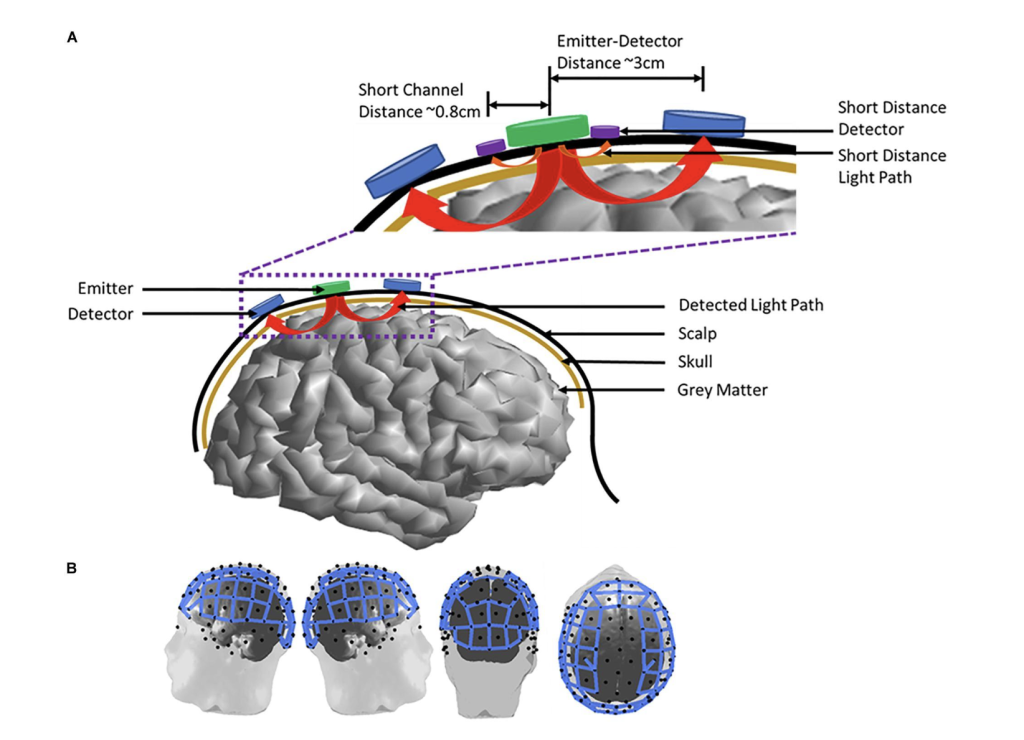
fNIRS is a non-invasive neuroimaging method used to measure cortical activation and monitor brain function in real time. It works by detecting changes in oxygenated (HbO2) and deoxygenated hemoglobin (HbR), which reflect neural activity. Near-infrared light, typically in the 650–950 nm range, is emitted into the scalp by a light source. This light penetrates the head tissues—including the scalp, skull, and cortex and diffuses in a curved, “banana-shaped” path between the source and detector. As neural activity increases in a region, oxygenated blood flow rises, changing the light absorption pattern. By measuring these changes, fNIRS provides insight into dynamic brain function.
One of the key advantages of fNIRS is its portability and tolerance to motion, making it ideal for real-time brain monitoring during naturalistic tasks. Unlike fMRI or PET, it allows participants to move, speak, or even perform physical activities during data collection (Ferrari & Quaresima, 2012).
A review of 26 studies using fNIRS during tasks such as dual-tasking, speaking, and cycling demonstrated consistent increases in oxygenated hemoglobin and decreases in deoxygenated hemoglobin during and after exercise. These findings support the utility of fNIRS in capturing brain activation in real-world, movement-heavy contexts. The research also showed increased prefrontal cortex activation during motor and cognitive tasks, and suggested that acute physical activity may enhance cognitive performance.
Currently, fNIRS is widely used in laboratory research and clinical settings. It has shown promise in diagnosing and monitoring neurological conditions such as schizophrenia, depression, autism spectrum disorder, stroke recovery, and Alzheimer’s disease. Given its real-time data collection, cost-effectiveness, and flexibility, fNIRS has strong potential for use in dynamic, high-stakes environments like sports (Rahman et al., 2020).
Table 1. Comparative overview of neuroimaging techniques (PET, MEG, fMRI, EEG, fNIRS) highlighting dimensions relevant to sports neuroscience; portability, temporal resolution, spatial resolution, motion tolerance, invasiveness, and suitability for athletic environments.


Potential Applications in Sports
Athletes are required to make quick decisions, manage their fatigue, and maintain good focus in their sports settings, so capturing changes in the brain across these variables is crucial in optimizing performance and safety (Tan et al., 2019). Since fNIRS allows for studying real time changes in brain oxygenation, coaches should adjust training intensity based on the athletes’ cognitive fatigue. Furthermore, when an athlete undergoes a head injury, such as a concussion, it is important to maintain constant monitoring of the brain.
In a study, fNIRS were used in conjunction with a portable transcranial Doppler (TCD) ultrasound on MMA athletes who had sensor based mouth guards on. Its goal was to detect changes in their cerebral oxygenation and blood flow. There was a substantial increase in blood flow velocity (p=0.01), and enhanced oxygenation in the motor cortex (p=0.04) and prefrontal cortex (p=0.01). fNIRS can be successfully used in sports activity and can capture immediate hyperacute hemodynamic changes even if an athlete showed no initial symptoms (Grijalva et al., 2023). It is useful in detecting early brain issues and can help athletes manage concussions and make safer decisions on when they can return to play. Traumatic injuries can result in long term neurological effects, especially in contact sports, so it is vital to monitor them. Neurodegenerative diseases such as CTE can be a result of repeated concussions and subconcussive impacts. When tau protein deposits abnormally in the frontal lobes, that’s when CTE pathology forms. There are many studies that include evidence of retired contact sport athletes such as boxers and football players that have memory issues, depression, suicidal behaviors, and dementia (Ling et al., 2015). By using the correct neuroimaging technique to monitor the symptoms and recovery of the athletes, like fNIRS, such problems could have been detected early on instead of allowing the athletes to further expose themselves in dangerous situations while continuing to play and evading the consequences of unmonitored brain trauma.
Currently, there is an effort by the U.S. Army Research Lab’s Translational Neuroscience Branch that aims to create wearable neuroimaging devices and integrate multiple sensors that can be used by non-specialists, like athletes. Additionally, there are many prototypes of wearable, high-resolution technologies that are able to collect sufficient data outside of the lab setting, demonstrating its successful real-world applications (McDowell et al., 2013).
Challenges and Limitations
Despite the numerous benefits to using fNIRS, there are cons to using this method. fNIRS have limited depth, as it can only capture signals from the cortical surface. Penetration of light is restricted to 1-3 centimeters into the cortex, which is not beneficial when monitoring subcortical areas. This restricts studying of motor coordination and emotional regulation, as well as the scope of data and analysis in a sports setting. Furthermore, optodes are usually spaced about 3 centimeters apart, leading to sparse spatial resolution, and makes precise localization of activation zones difficult. This can disproportionally affect data reliability (Huo at al., 2024).
Additionally, hair and scalp interference is a major issue because hair texture, thickness, color, and skin pigmentation can affect signal absorption and scattering. A study concluded that participants who had thicker hair, curly hair, or higher scalp melanin concentrations demonstrated significant decreases in signal-to-noise ratio and increased light attenuation. The article suggests using brush-fiber optodes. Parting and braiding hair, recording detailed metadata on hair and skin for quality assessment, and making individualized cap fitting and placement (Yücel et al., 2024). Without such accommodations, fNIRS data sets risk bias by misrepresenting certain demographic groups, which is not appropriate for diverse sports settings.
Future Directions
Emerging wearable technologies are starting to revolutionize the scalability of fNIRS. For this methodology to continue advancing, correcting skull/scalp bloodflow/systematic effects by equipping them with different source-detector distances is necessary, as well as maintaining consistent protocols and signal correction methods for clinical grade results (Jounghani et al., 2024). TD-FNIRS are still being further miniaturized and their signal-to-noise ratio is constantly being improved. Wearable and low cost bio sensors are also becoming more compact and advanced. Additionally, with high-density optode rays and more advanced signal processing, the spatial resolution of fNIRS can be significantly improved and can help distinguish brain signals from other interferences, thus increasing data accuracy and broadening applicability of fNIRS (Quaresima & Ferrari et al., 2019).
Recently, researchers from both Vanderbilt University and Stanford University School of Medicine created a low cost, wearable fNIRS system (Rahimpour Jounghani et al., 2025). It is fully integrated and wireless, allowing for brain monitoring in everyday settings. The headband has a single LED-pair light source and 4 detectors, while prioritizing comfort and easy use. Its 2 LEDs have near-infrared wavelengths at 740 nm and 850 nm. NIRDuino is open-source Android configurable, modular, and is Bluetooth enabled allowing for user-friendly brain monitoring (Kumar et al., 2024). Future models may possess up to 10 detectors with multiple light sources. Such a development opens up avenues for research in many fields as well as enabling brain monitoring in naturalistic environments. These innovations can make their way into large-scale-athlete monitoring and other non-clinical settings due to its many benefits.
Conclusion
Functional near-infrared spectroscopy represents a significant step forward in bridging neuroscience and athletic performance. Unlike PET, MEG, or fMRI, which are hindered by their lack of portability and susceptibility to motion, fNIRS enables real-time monitoring of brain activity in naturalistic sports environments. It can assess cognitive workload, fatigue, decision-making, and recovery, providing valuable information for optimizing training and supporting safer return-to-play protocols. Although challenges remain—including limited cortical depth and susceptibility to hair and scalp interference—technological advances are steadily expanding the scope and precision of fNIRS. Innovations such as wearable wireless devices, improved sensor arrays, and machine learning–based analysis point toward a future where large-scale, field-based monitoring of athletes is feasible. By enhancing both performance and safety, fNIRS has the potential to transform sports neuroscience and contribute meaningfully to the long-term health and success of athletes.
References
Baillet, S. (2017). Magnetoencephalography for brain electrophysiology and imaging. Nature neuroscience, 20(3), 327-339.
Chen, W. L., Wagner, J., Heugel, N., Sugar, J., Lee, Y . W., Conant, L., … & Whelan, H. T. (2020). Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: advances and future directions. Frontiers in neuroscience, 14, 724.
Cherry, S. R. (2001). Fundamentals of positron emission tomography and applications in preclinical drug development. The Journal of Clinical Pharmacology, 41(5), 482-491.
Ferrari, M., & Quaresima, V . (2012). A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage, 63(2), 921-935.
Grijalva, C., Mullins, V . A., Michael, B. R., Hale, D., Wu, L., Toosizadeh, N., … & Laksari, K. (2023). Neuroimaging, wearable sensors, and blood-based biomarkers reveal hyperacute changes in the brain after sub-concussive impacts. Brain multiphysics, 5, 100086.
Huo, C., Xu, G., Xie, H., Chen, T., Shao, G., Wang, J., … & Li, Z. (2024). Functional near-infrared spectroscopy in non-invasive neuromodulation. Neural Regeneration Research, 19(7), 1517-1522.
Jounghani, A. R., Gozdas, E., Dacorro, L., Avelar-Pereira, B., Reitmaier, S., Fingerhut, H., … & Hosseini, S. H. (2024). Neuromonitoring-guided working memory intervention in children with ADHD. iScience, 27(11).
Kumar, A., Crawford, S., Le, T. C., Jounghani, A. R., Carbonell, L. M., Capps, A. S., … & Bowden, A. K. (2024). NIRDuino: A modular, Bluetooth-enabled, Android®-configurable fNIRS system with dual-intensity mode built on Arduino®. medRxiv, 2024-12.
Ling, H., Hardy, J., & Zetterberg, H. (2015). Neurological consequences of traumatic brain injuries in sports. Molecular and Cellular Neuroscience, 66, 114-122.
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453(7197), 869-878.
Quaresima, V ., & Ferrari, M. (2019, August). A mini-review on functional near-infrared spectroscopy (fNIRS): where do we stand, and where should we go?. In Photonics (V ol. 6, No. 3, p. 87). MDPI.
Rahimpour Jounghani, A., Kumar, A., Moreno Carbonell, L., Aguilar, E. P. L., Picardi, T. B., Crawford, S., … & Hosseini, S. H. (2025). Wearable fNIRS platform for dense sampling and precision functional neuroimaging. npj Digital Medicine, 8(1), 271.
Rahman, M. A., Siddik, A. B., Ghosh, T. K., Khanam, F., & Ahmad, M. (2020). A narrative review on clinical applications of fNIRS. Journal of Digital Imaging, 33(5), 1167-1184.
McDowell, K., Lin, C. T., Oie, K. S., Jung, T. P., Gordon, S., Whitaker, K. W., … & Hairston, W. D. (2013). Real-world neuroimaging technologies. Ieee Access, 1, 131-149.
Tan, S. J., Kerr, G., Sullivan, J. P., & Peake, J. M. (2019). A brief review of the application of neuroergonomics in skilled cognition during expert sports performance. Frontiers in human neuroscience, 13, 278.
Yücel, M. A., Anderson, J. E., Rogers, D. J., Hajirahimi, P., Farzam, P., Gao, Y ., … & Boas, D. A. (2024). Quantifying the Impact of Hair and Skin Characteristics on Signal Quality with Practical Recommendations for Improvement. bioRxiv.
About the author

Vanessa Georgieva
Vanessa Georgieva is a 12th grader at Hinsdale Central Highschool. She has a passion for biomedical engineering and is also a figure skater and coach, which are her extracurricular passions. This motivated her to position her paper into a view on fNIRS impacts in sports environments.