
Author: Vyomesh Vikram Singh
Mentor: Dr. Zion Tse
City Montessori School
Abstract
Human–computer interfaces represent a rapidly advancing frontier in biomedical engineering, integrating mechanical, electronic, neural, and Artificial Intelligence (AI) technologies to restore or augment lost human function. This review synthesizes recent developments in Advanced Human–Computer Interfaces, organs, and neural interfaces, drawing on both clinical and engineering perspectives. With a focus on state-of-the-art innovations published within the last five years, the paper highlights breakthroughs in AI-driven sensory feedback, adaptive control algorithms, biomaterials, and clinical translation. By situating these advances within the broader context of unmet clinical needs and rehabilitation goals, this review identifies current challenges and outlines future directions for fully integrated, intelligent human-machine systems.
Index Terms
Machine learning, Deep learning, Reinforcement learning, Edge computing, On-device AI, Computer vision, Bionic limbs, brain–computer interfaces, prosthetics, neuromorphic vision, osseointegration, artificial pancreas, neural interfaces, Advanced Human–Computer Interfaces, AI, biomedical engineering, neuroprosthetics, human–machine symbiosis, wearable robotics, neuroengineering, biocompatible materials, smart prosthetics, adaptive control, closed-loop systems, neural decoding, assistive technology, implantable devices, bioelectronics, translational medicine, cybernetics.
I. Introduction
The pursuit of artificial devices that restore lost biological function is as old as medicine itself, with early wooden prosthetic legs and iron hooks marking humanity’s first attempts at bionics. In the modern era, bionic devices have come to represent a class of technologies that combine mechanical hardware, electronic control, and neural interfacing to restore sensory, motor, or organ-level function. These devices are no longer crude substitutes; rather, they aim for seamless integration with the human nervous system, allowing users to experience levels of dexterity, feedback, and autonomy once thought impossible.
The importance of this field is underscored by the prevalence of disability worldwide. According to the World Health Organization, over 2.4 billion people globally live with conditions requiring rehabilitation [21]. Of these, limb loss affects more than 57 million people, while vision loss (addressable by devices like the bionic eye) impacts at least 43 million blind individuals [21]. In diabetes alone, more than 530 million people require continuous glucose management, and the artificial pancreas is emerging as a transformative bionic organ [23]. These statistics highlight the vast unmet need that bionic technologies attempt to address.
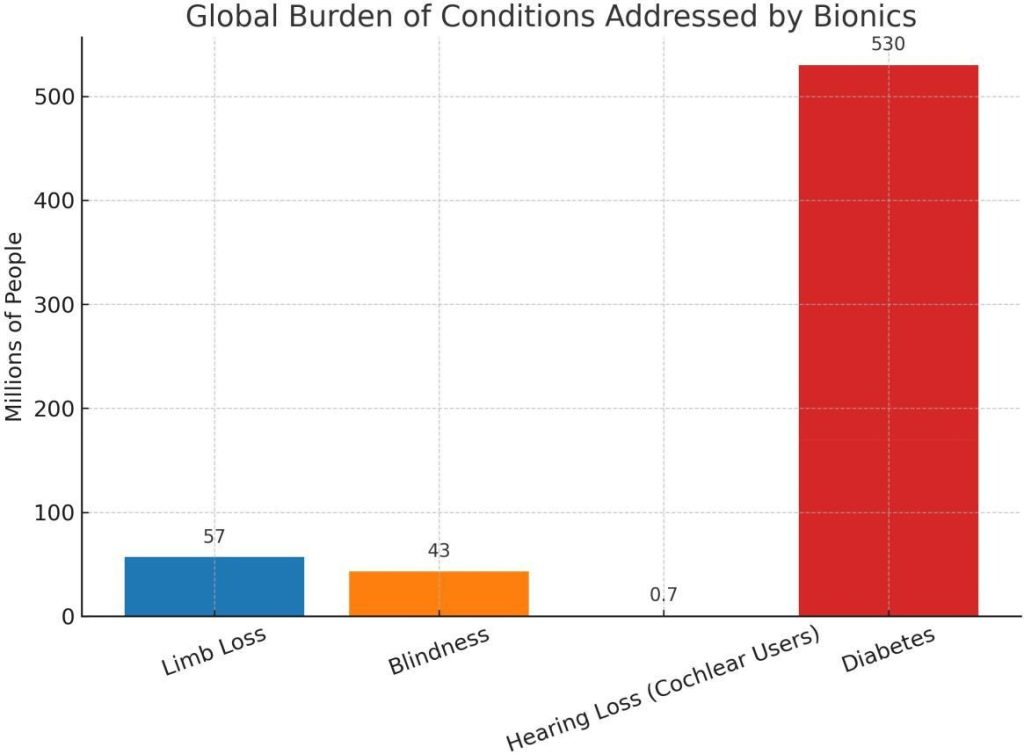
Fig. 1: Global burden of conditions addressed by bionics. Bars show approximate affected populations: limb loss (57M), blindness (43M), cochlear implant users (∼0.7M), and diabetes (530M). Data sources: WHO Global Report on Rehabilitation [21] (limb loss, blindness, diabetes) and Wilson (cochlear implant users) [24].
II. Overview of Human-Computer Interfaces
Human–Computer Interfaces (HCIs) can be defined as artificial constructs designed to replace or augment biological structures, with the unique feature of neural, physiological, and increasingly AI-driven integration. Unlike conventional prosthetics or implants that function passively, HCIs actively sense, compute, learn, and actuate.
Historically, the field has undergone several stages. Early prosthetics, such as Egyptian wooden toes or Roman iron hands, were primarily cosmetic or functional in the most basic sense. By the 16th century, artisans like Ambroise Paré introduced mechanical limbs with crude joint mechanisms. The 20th century saw the introduction of body-powered prostheses (using cables and harnesses), followed by the revolutionary myoelectric control in the 1960s, which used Electromyographic (EMG) signals for actuation [16]. In parallel, sensory HCIs began with the cochlear implant (1972), the first device to restore a lost sensory modality via direct neural stimulation, paving the way for retinal implants and, more recently, neuromorphic vision systems based on organic semiconductors and perovskite nanowire arrays [10], [11]. Organ-level HCIs have also advanced, most notably the artificial pancreas, which integrates glucose sensors, insulin pumps, and AI-based closed-loop metabolic control algorithms [23].
To situate the reader in the diversity of modern HCIs, Table I summarizes major categories of systems, their principles of operation, and representative examples. This overview highlights a unifying theme: HCIs are no longer restricted to mechanical substitution. Instead, modern systems seek bidirectional communication with the nervous system—allowing users not only to control artificial devices but also to receive naturalistic sensory feedback enhanced by adaptive AI.
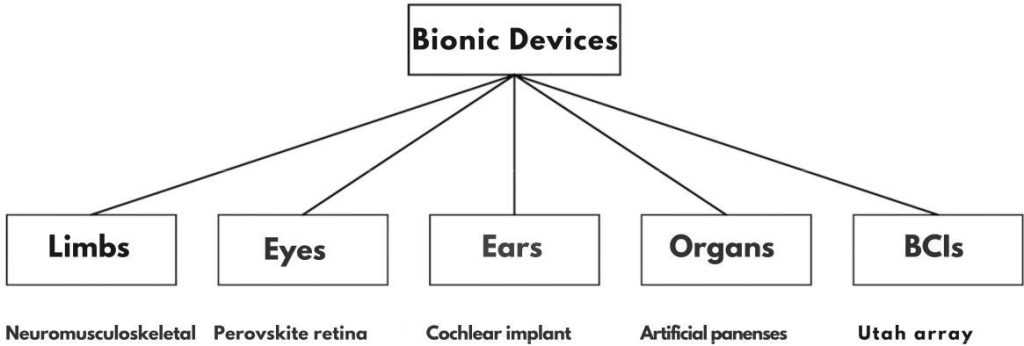
Fig. 2: Taxonomy of Human–Computer Interfaces (HCIs) across five categories: limbs [1], eyes [10], ears [22], organs [23], and brain–computer interfaces (BCIs) [14].
TABLE I: Major Classes of Bionic Devices, Principles, and Examples
| Category | Principle of Operation | Key Examples | Representative | ||
| Bionic Limbs | Capture myoelectric/neuralsignals; actuate robotic joints; provide sensory feedback via electrodes/sensors | NeuromusculoskeletalProsthesis; bidirectional limb; targeted reinnervation systems | Ortiz-Catalan et al.(2023) [1], Marascoet al. (2021) [3] | ||
| Bionic Eye | Convert light into electrical signals processed by neuromorphic/electrode arrays interfacing with retina or optic nerve | Argus II retinal prosthesis;perovskite nanowireRetina; TIPS-pentacene retina | Long et al.(2023) [10], Zhanget al. (2023) [11] | ||
| Bionic Ear | Convert sound into electricalimpulses transmitted viacochlear electrodes | Cochlear implant | Loizou (2006) [22],Wilson (2017) [24] | ||
| Bionic Organs | Closed-loop sensing andactuation replacing organ-level function | Artificial pancreas; bioartificial heart pumps | Hovorka (2011) [23],Breton (2019) | ||
| Neural Interfaces / Brain Computer Interfaces (BCIs) | Decode brain or peripheralnerve activity to controlexternal devices; deliverstimulation for feedback | Utah array BCIs; Regenerative peripheral nerve interfaces (RPNIs) | Hochberg et al.(2012) [14], Cho etal. (2023) [7] |
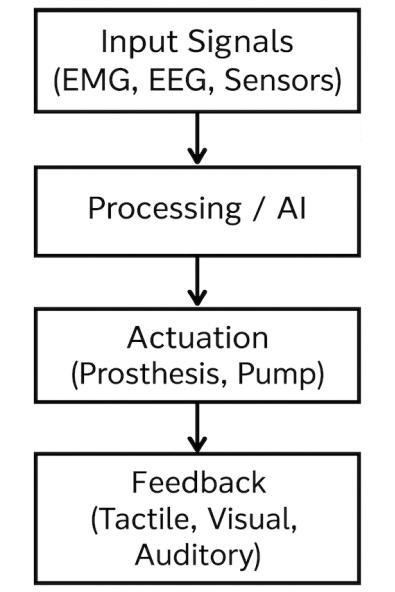
Fig. 3: Functional pipeline of a bionic device: input signals (e.g., EMG/EEG/sensors) → processing/AI → actuation (prosthesis/pump) → feedback (tactile/visual/auditory).
III. Current Types of Human-Computer Interfaces
HCIs span a wide range of applications, from motor prostheses that restore limb function to sensory prostheses that recreate lost modalities such as hearing and vision. In addition, organ-level HCIs represent an emerging frontier where closed-loop control systems, often enhanced by AI, substitute for failing metabolic or physiological functions. In this section, we review the major classes of HCIs, focusing on their principles of operation, technological foundations, and representative studies from a span of five years. Throughout this review, we use Human–Computer Interfaces (HCIs) as the overarching term for technologies that bridge biological and computational systems. Terms such as bionic limbs, bionic eyes, and related phrases are used to denote specific subsets of HCIs, rather than distinct categories.
A. Bionic Limbs
Upper-Limb Prostheses: Upper-limb prostheses have progressed from simple hooks to highly dexterous, multi-articulated robotic hands with neural control. The control of such devices primarily relies on myoelectric signals derived from the residual muscles of the forearm or upper arm. However, conventional surface EMG suffers from poor signal quality, cross-talk, and electrode displacement. To overcome these limitations, modern systems employ implanted electrodes (epimysial, intramuscular) that record stable myoelectric activity over years [1].
1. Advanced interfaces include Targeted Muscle Reinnervation (TMR) and Regenerative Peripheral Nerve Interfaces (RPNIs). TMR surgically reroutes residual nerves to denervated muscles, creating new, amplifiable EMG sites [16]. RPNIs implant nerve endings into muscle grafts, forming stable bioelectrical sources for long-term control [7]. These constructs amplify weak nerve signals into robust EMG activity, enabling fine motor decoding through pattern recognition or regression algorithms.
Recent clinical translation is exemplified by Ortiz-Catalan et al. , who demonstrated a transradial neuromusculoskeletal prosthesis integrating osseointegrated titanium implants, implanted electrodes, and neural stimulation [1]. The patient achieved stable prosthetic use in daily life for more than three years,
TABLE II: Representative Advances in Bionic Limbs
| Study / Device | Key innovation | Control Strategy | Sensory Feedback | Clinical Outcome |
| Ortiz-Catalan et al. (2023) [1] | OsseointegratedNeuromusculo-skeletal prosthesis | ImplantedEMG (native +graft) | Ulnar nerve cuff, tactile feedback | >3 years daily use;Pain reduction |
| Marasco et al. (2021) [3] | Fusion of touch, kinesthesia, and motor control | TMR + TSR EMG | Kinesthetic +Tactile reinnervation | Able-bodied visuomotor behaviours |
| Open Source Leg (2020) [6] | Modular, programmable powered leg | Adaptive gait control | Not integrated | Clinical testing in transfemoral amputees |
| BeBionic / i-Limb (commercial) | Multiarticulated commercial hands | Surface EMG,Pattern recognition | Limited vibrotactile feedback | Widely available, limited embodiment |
with reduced phantom limb pain and improved quality of life. This represents one of the first long-term demonstrations of a self-contained neural prosthesis outside the laboratory.
In addition to control, sensory feedback has become a critical area of research. Extraneural cuff electrodes and intraneural arrays can evoke tactile percepts in the phantom limb when coupled with sensorized prosthetic hands [2]. Marasco et al. further demonstrated the fusion of touch, kinesthesia, and motor control, restoring able-bodied visuomotor behaviors such as reducing visual fixation on the prosthetic hand [3]. These findings suggest that upper-limb prostheses are approaching a new level of embodiment and naturalistic use.
2. Lower-Limb Prostheses: Lower-limb bionics are distinguished by the need to restore both mobility and load-bearing stability. Microprocessor-controlled knees (e.g., Ottobock C-Leg, Össur Rheo Knee) represent the current standard of care, providing adaptive damping based on gait phase detection. Recent developments extend this paradigm to powered prosthetic legs, which incorporate actuators at the knee and ankle.
The Open Source Leg (OSL) is a notable example, offering a modular, customizable platform with open hardware and software [6]. Clinically tested on transfemoral amputees, the OSL provides knee and ankle actuation with programmable gait dynamics. This democratized design accelerates innovation by lowering barriers for academic and clinical groups to experiment with novel control strategies.
3. Osseointegration and Direct Skeletal Attachment: Conventional socket-based attachment causes discomfort, skin breakdown, and instability. Osseointegration—anchoring the prosthesis directly to the skeleton via titanium implants—addresses these issues [15]. Beyond mechanical benefits, osseointegration serves as a human–machine gateway, enabling safe percutaneous feedthroughs for electrodes and sensors. This transforms the limb into a bidirectional interface, capable of both decoding neural intent and delivering somatosensory feedback [1].
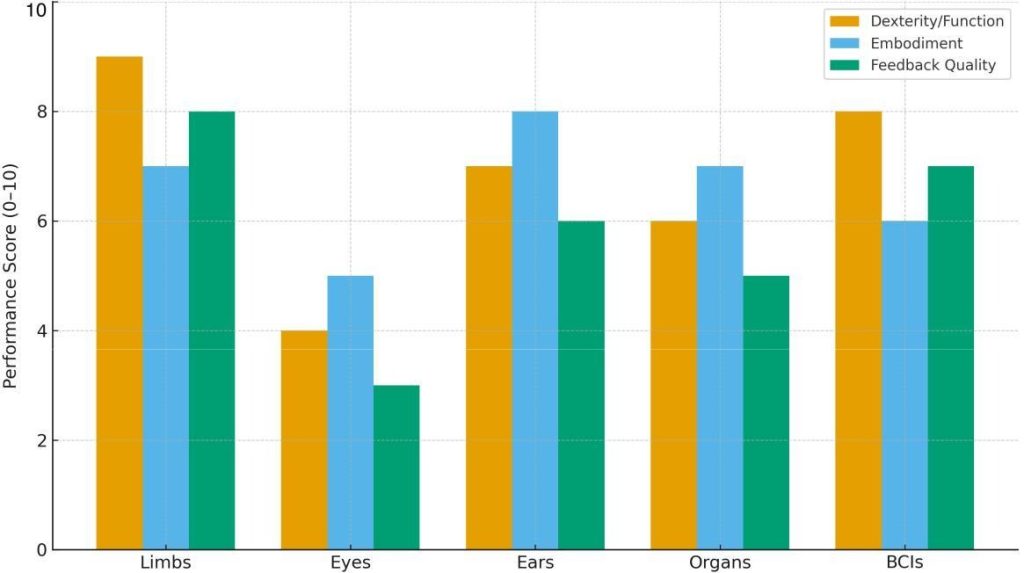
Fig. 4: Comparative performance metrics across device classes (illustrative 0–10 scores). Values synthesize trends reported in representative studies on neuromusculoskeletal limbs [1], [3], bionic vision and retinal systems [5], [10], [11], cochlear implants [22], [24], artificial pancreas systems [23], and clinical/BCI demonstrations [13], [14].
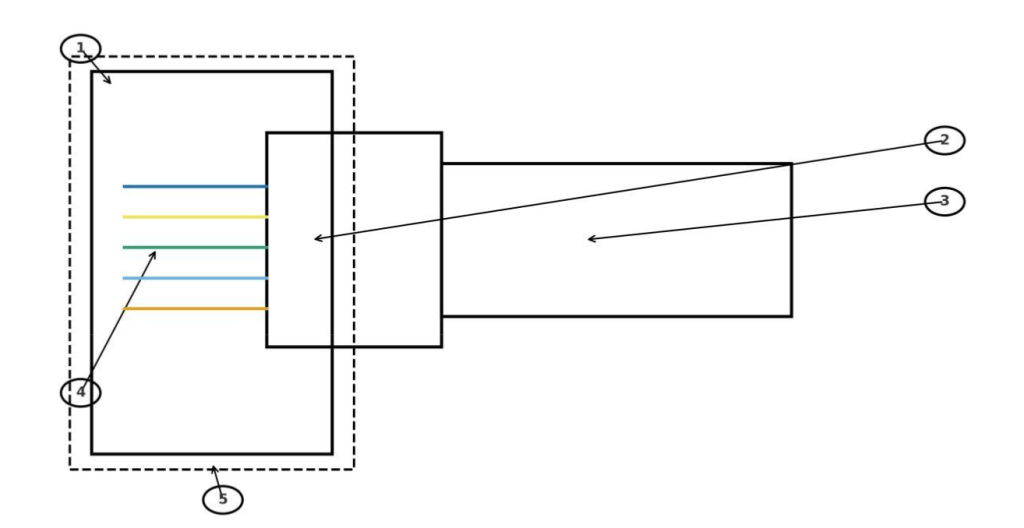
Fig. 5: Case study schematic of osseointegration and neural interfaces in human–computer interface (HCI) limbs. The diagram shows bone anchoring, titanium implant, prosthesis connector, electrodes to nerve/muscle, and percutaneous feedthroughs. Legend: (1) Bone, (2) Titanium implant, (3) Prosthesis connector, (4) Electrodes to nerve/muscle, (5) Skin layer (dashed).
TABLE III: Recent Innovations in Bionic Vision
| Device / Material | Key Feature | Advantage |
| Argus II (Second Sight) | Retinal electrode array | Restores basic light perception |
| Long et al. (2023) | Perovskite nanowire retina | Filter-free color vision; wide FoV |
| Zhang et al. (2023) | TIPS-pentacene organic retina | Broadband; synaptic plasticity |
| Artificial Synapse Retinas | Memristor-based photonic synapses | In-device preprocessing; memory |
B. Bionic Eyes
The restoration of vision is among the most ambitious goals of sensory prosthetics. Early retinal implants such as Argus II used electrode arrays to stimulate the surviving retinal ganglion cells, enabling basic light perception and object localization [5]. However, spatial resolution remained low, and the devices were limited to high-contrast vision.
Recent approaches leverage neuromorphic engineering and novel materials. Long et al. reported a hemispherical perovskite nanowire retina capable of filter-free color recognition [10]. By integrating adaptive optics with neuromorphic preprocessing circuits, the system achieved wide-field, low-noise, and low-power color vision. Similarly, Zhang et al. developed a TIPS-pentacene phototransistor retina, exhibiting broadband sensitivity (380–740 nm), high optical transparency, and synaptic plasticity for visual memory [11]. The choice of TIPS-pentacene, with its narrow bandgap (∼1.6 eV), enabled efficient photon absorption across the visible spectrum, mimicking natural photoreceptors.
These neuromorphic systems go beyond electrode-based stimulation by embedding preprocessing within the retina itself, thereby reducing latency and power consumption. While still in preclinical stages, they represent a paradigm shift toward bioinspired vision systems capable of continuous learning and adaptation.
C. Bionic Ears
The cochlear implant remains the most successful sensory prosthesis to date, with more than 700,000 users worldwide [22]. It works by bypassing damaged hair cells of the cochlea and directly stimulating the auditory nerve with electrode arrays. Modern cochlear implants use advanced signal processing algorithms to decompose sound into frequency channels and deliver spatially coded electrical impulses.
Key technological progress includes fine structure processing, which encodes temporal cues for improved music perception, and optogenetic cochlear implants, which use light to stimulate genetically modified neurons with higher precision [24]. While conventional devices are limited to about 22 electrode channels, optogenetic approaches promise higher resolution with reduced channel interaction.
D. Bionic Organs
- Artificial Pancreas: The artificial pancreas integrates a Continuous Glucose Monitor (CGM) with an insulin pump under closed-loop algorithmic control. Early systems used Proportional–Integral–Derivative (PID) control, but modern devices employ Model Predictive Control (MPC), which anticipates glucose fluctuations based on meals, exercise, and circadian rhythms [23]. Clinical trials show that MPC-based artificial pancreas systems reduce hypoglycemia incidence and improve HbA1c compared to conventional insulin therapy.
- Other Organ-Level Devices: Beyond diabetes, prototypes of bionic kidneys (artificial filtration units) and bioartificial hearts are under investigation. For example, wearable dialysis systems combine nanoporous membranes with microfluidic pumps, while ventricular assist devices integrate soft robotics for pulsatile flow. While less mature than limb or sensory prostheses, these devices extend the concept of bionics to systemic organ replacement.
E. Neural Interfaces and Brain–Computer Interfaces
Neural interfaces serve both as standalone assistive technologies and as enabling components of bionic limbs and sensory devices. They can be broadly classified as non-invasive (EEG, fNIRS), minimally invasive (ECoG), and invasive (Utah arrays, intraneural electrodes).
Non-invasive BCIs offer safety and accessibility but suffer from low spatial and temporal resolution. Invasive approaches achieve higher bandwidth but face challenges of biocompatibility and stability. Recent advances include:
- Hybrid nerve interfaces (Cho et al., 2023) that combine RPNIs with shape-memory polymer buckles, achieving stable bidirectional signaling in animal models [7].
- Reinforcement learning-based BCIs that improve prosthetic hand control accuracy by double to triple compared to supervised learning [12].
- Bidirectional BCIs that not only decode motor intent but also deliver sensory feedback through cortical stimulation [13], [14].
These technologies are converging toward closed-loop systems, where intention and perception are integrated within the same neural–machine cycle.
IV. Technological Advancements and State-of-the-Art
The performance of bionic devices is fundamentally constrained by the quality of their materials, sensors, actuators, and neural interfacing technologies. In recent years, breakthroughs in biocompatible materials, microelectronics, and artificial intelligence have dramatically improved the fidelity of motor control, the richness of sensory feedback, and the long-term stability of implantable systems. This section reviews these advances, emphasizing both the underlying mechanisms and the way they address prior limitations.
A. Materials for Bionics
1. Biocompatible Polymers and Flexible Electronics: Traditional rigid electronics are poorly matched to the soft, dynamic environment of biological tissue, often leading to inflammatory responses and signal degradation. Flexible polymers such as polyimide, PDMS (polydimethylsiloxane), and parylene-C have become standard substrates for implantable electrodes. These materials reduce mechanical mismatch, minimizing scar tissue encapsulation and improving long-term signal stability [9].
Emerging organic semiconductors have further enabled neuromorphic HCIs. For instance, 6,13-bis (triisopropylsilylethynyl)pentacene (TIPS-pentacene) was selected in Zhang et al. for its narrow bandgap (∼1.6 eV), which allows photon absorption across the visible spectrum [11]. Its high carrier mobility and optical transparency made it ideal for constructing a retina-like phototransistor array that mimics the broadband response of photoreceptors. TIPS-pentacene also exhibits synaptic plasticity under repeated light stimulation, enabling short-term visual memory tasks and demonstrating how material choice directly determines device intelligence. In short, TIPS-pentacene functions as a photoactive organic semiconductor that provides both light detection and learning-like behavior within neuromorphic retinal systems.
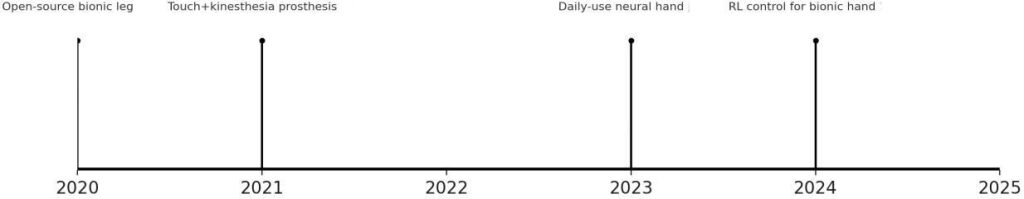
Fig. 6: Innovation timeline (2020–2025) highlighting selected advances from the reference set. Key milestones include the open-source bionic leg (2020) [6], prosthetic touch and kinesthesia integration (2021) [3], a clinically deployed neural-controlled bionic hand (2023) [1], and reinforcement learning control for dexterous hand function (2024) [12].
2. Metals and Ceramics for Osseointegration: In HCI limbs, titanium alloys are the gold standard for osseointegration due to their high strength, corrosion resistance, and biocompatibility. Surface treatments (e.g., hydroxyapatite coatings) promote bone ingrowth, enabling long-term skeletal anchoring [15]. The percutaneous feedthroughs enabled by titanium implants also provide a stable, infection-resistant pathway for electrodes, addressing the long-standing problem of percutaneous connectors in neural prostheses.
B. Sensors and Actuators
Bionic devices rely on sensors to detect environmental stimuli and actuators to reproduce biological motion.
- MEMS and Soft Sensors: Miniaturized Microelectromechanical Systems (MEMS) enable high-resolution detection of forces, pressures, and accelerations. In prosthetic hands, MEMS pressure sensors embedded in fingertips translate tactile stimuli into electrical signals that can be delivered back to the nervous system [3]. For lower-limb devices, Inertial Measurement Units (IMUs) allow real-time detection of gait phases, enabling adaptive damping in microprocessor knees.
Soft sensors based on conductive hydrogels or liquid metals are increasingly integrated into prosthetic liners, providing conformal detection of skin strain and residual limb pressure. These sensors improve socket fit monitoring and help prevent skin breakdown.
- Actuation Systems: Historically, prosthetic actuation relied on DC motors, which are bulky and energy-inefficient. Recent approaches explore series elastic actuators, which integrate elastic elements to store and release energy, improving safety and compliance during human–robot interaction. Shape Memory Alloys (SMAs) and Dielectric Elastomer Actuators (DEAs) offer biomimetic muscle-like contraction, although their power efficiency and thermal properties remain challenges.
In the context of the artificial pancreas, actuators are miniaturized insulin pumps capable of delivering subcutaneous doses with millisecond precision. The accuracy of these pumps, combined with continuous glucose monitoring, underpins the safety of closed-loop systems [23].
C. Neural Interfaces
The neural interface is the critical bottleneck for high-performance bionic systems, as it governs the bandwidth of communication between the user and the device.
- Non-Invasive vs. Invasive Interfaces: Non-invasive techniques such as surface EMG and EEG (Electromyography and Electroencephalography) are safe but limited by poor signal-to-noise ratio. In contrast, invasive methods such as intraneural electrodes, epimysial implants, and cortical arrays offer high bandwidth but risk tissue damage and long-term instability.
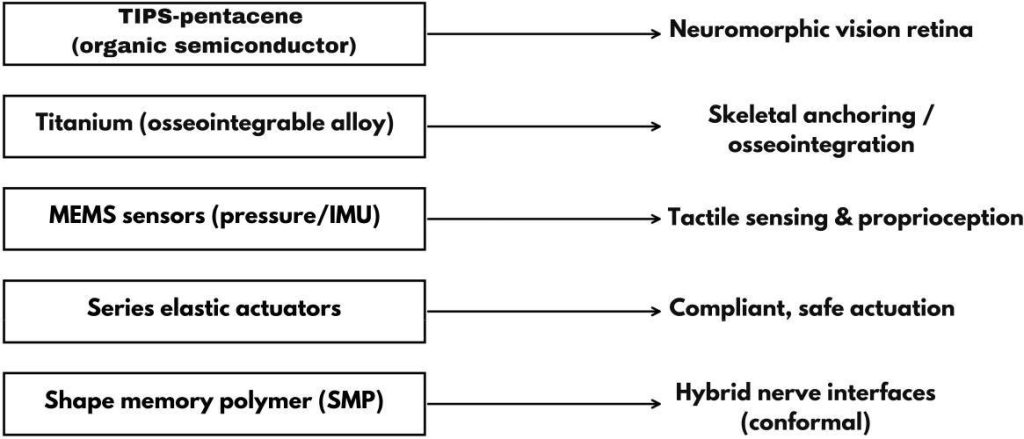
Fig. 7: Material-to-function mapping: examples include TIPS-pentacene → neuromorphic vision; titanium → osseointegration; MEMS → tactile/proprioception; series elastic actuators → compliant actuation; shape-memory polymers (SMP) → hybrid nerve interfaces.
The hybrid nerve interface proposed by Cho et al. exemplifies a middle ground [7]. By combining regenerative peripheral nerve interfaces (muscle grafts reinnervated by nerve endings) with a shape memory polymer buckle, the system stabilized nerve–electrode contact over 29 weeks in rabbits. This design achieved stable bidirectional communication—demonstrating both sensory recording and robotic leg control—suggesting that hybrid constructs may resolve the trade-off between invasiveness and stability.
- Osseointegration as a Neural Gateway: Ortiz-Catalan et al. demonstrated that osseointegrated implants could act not only as skeletal anchors but also as percutaneous conduits for neural signals [1]. By routing electrode wires through titanium fixtures integrated into the radius and ulna, they achieved stable myoelectric recording and direct neural stimulation for more than three years in daily use. This dual role of osseointegration—as both a mechanical interface and a neural gateway—represents a major advance in long-term clinical viability.
D. Computational Algorithms and Decoding
Advances in machine learning have transformed bionic control from binary, sequential commands to rich, continuous decoding of intent.
- Pattern Recognition and Regression: Early myoelectric prostheses employed simple thresholding: one muscle contraction to open the hand, another to close. Modern devices employ pattern recognition algorithms (support vector machines, linear discriminants) trained on multichannel EMG to decode a variety of gestures. Regression-based approaches enable proportional control, translating EMG amplitude directly into joint torque or velocity.
- Reinforcement Learning (RL): A major innovation is the use of reinforcement learning for prosthetic control. Schone et al. implemented an RL framework with a “Guitar Hero”-like training game, where users received real-time feedback as they attempted specific hand gestures [12]. Over time, the system adapted both to user variability and electrode drift. The RL-based controller achieved double to triple the accuracy of supervised learning, particularly for simultaneous multi-finger movements. This demonstrates how adaptive algorithms can overcome the limitations of static calibration
TABLE IV: Technological Innovations Underpinning Modern Bionic Devices
| Domain | Innovation | Key Mechanism /Material | Problem Solved | Representative Study | |
| Materials | TIPS-pentacenephototransistors | Narrow bandgap organic semiconductor | Broadband visibleabsorption; synaptic plasticity | Zhang | Zhang et al.(2023) [11] |
| Materials | Titanium osseointegration | Biocompatible alloy with bone integration | Stable skeletalanchoring; neuralfeedthrough | Ortiz-Catalan et al.(2023) [1] | |
| Sensors | MEMS tactile arrays | Miniaturized pressure sensors | High-resolutiontouch feedback | Marasco et al.(2021) [3] | |
| Actuators | Series elastic actuators | Elastic compliance elements | Safe interaction;energy efficiency | Clites | Clites et al.(2020) [6] |
| Neural Interfaces | Hybrid nerve interface + SMP buckle | Muscle graft +shape memorypolymer | Stable long-termnerve–electrodecontact | Cho | Cho et al.(2023) [7] |
| Algorithms | Reinforcement learning control | Adaptive policy optimization | Improved multi-DoF accuracy | Schone et al.(2024) [12] | |
| Algorithms | Memristor neuromorphic retina | Non-volatile resistive elements | Low-powerin-sensorpreprocessing | Long | Long et al.(2023) [10] |
- Neuromorphic and Memristor-Based Processing: Neuromorphic circuits emulate biological synapses and neurons in hardware. Memristors—resistive devices whose state depends on prior activity—are well-suited for synaptic plasticity. In bionic vision, memristor arrays handle in-sensor preprocessing, filtering noise and adapting to light without external processors, reducing latency and power use. Their non-volatility and scalability enable compact, low-power integration on flexible substrates. A neuromorphic retina can thus perform edge detection or motion tracking directly, similar to how biological retinas preprocess visual input.
E. Integration of Artificial Intelligence
Artificial intelligence (AI) extends beyond decoding to the holistic control of closed-loop systems. In the artificial pancreas, AI-based controllers predict insulin needs using historical glucose patterns, exercise levels, and meal timing [23]. In bionic limbs, deep learning models can classify EMG signals in real time, while reinforcement learning adapts to new conditions without retraining, continuously improving performance over extended use.
The integration of AI also facilitates user-specific personalization. Each patient’s physiology, residual limb anatomy, and lifestyle are unique; AI enables prostheses to learn individual preferences, adjust grip force for common tasks, anticipate fatigue, or predict gait transitions under different terrains. Such adaptive capabilities reduce cognitive burden on the user and improve naturalistic embodiment of the device.
As computing hardware becomes more compact and energy-efficient, these algorithms are increasingly embedded on-device, reducing latency and dependence on external computers. This mirrors the neural efficiency of biological systems, which integrate sensing, computation, and actuation locally. Looking ahead, the convergence of AI with neuromorphic hardware and flexible bioelectronics promises to create prostheses that operate autonomously, respond seamlessly to environmental changes, and evolve alongside the user’s daily needs.
V. Clinical Applications and Outcomes
The ultimate measure of success for any bionic technology lies not in laboratory demonstrations but in clinical effectiveness and real-world adoption. In this section, we examine the outcomes of bionic limbs, sensory prostheses, and organ-level devices in patients. This section emphasizes rehabilitation results, usability, quality of life improvements, and challenges revealed in long-term use.
A. Bionic Limbs in Clinical Use
- Upper-Limb Prostheses: Clinical trials of advanced upper-limb prostheses have demonstrated functional restoration, reduction in phantom limb pain, and increased quality of life. Ortiz-Catalan et al. reported a transradial neuromusculoskeletal prosthesis used continuously for more than three years [1]. Functional scores improved significantly: Southampton Hand Assessment Procedure (SHAP) scores increased by 23%, while pain interference with daily life decreased by more than 50%. Importantly, the user reported being able to wear the device comfortably throughout the day, an outcome rarely achieved with socket-based systems.
Marasco et al. studied two patients with targeted reinnervation and closed-loop feedback [3]. Integration of touch, kinesthesia, and motor control allowed participants to perform tasks with visuomotor behaviors indistinguishable from able-bodied individuals. They no longer had to fixate visually on the prosthetic hand, freeing attention for higher-level planning. This demonstrates not only functional restoration but also a shift toward naturalistic embodiment. - Lower-Limb Prostheses: Lower-limb prostheses are judged by metrics such as walking speed, energy expenditure, and stability on varied terrain. Microprocessor knees consistently improve gait symmetry and reduce falls compared to mechanical knees [6]. Powered prostheses such as the OSL enable active ankle push-off, reducing metabolic cost of walking. Early clinical trials show transfemoral amputees can achieve walking speeds approaching those of able-bodied controls.Osseointegrated lower-limb prostheses also demonstrate marked improvements in mobility. A long-term Swedish cohort study showed that more than 90% of patients reported improved prosthetic comfort, stability, and walking endurance compared to sockets [15]. However, risks such as infection and implant loosening persist.
B. Bionic Eyes
Retinal prostheses such as Argus II have provided basic functional vision to hundreds of blind individuals worldwide [5]. Clinical outcomes show patients can detect light sources, navigate high-contrast environments, and recognize large objects. However, resolution is limited (about 60 electrodes), and many users report “pixelated” vision.
More recent neuromorphic retinal devices remain at the preclinical stage but show transformative potential. Long et al. demonstrated that a perovskite nanowire retina achieved filter-free color discrimination and wide-field imaging in laboratory models [10]. Similarly, Zhang et al. reported that their organic phototransistor retina exhibited plasticity and visual memory, features that could translate to more naturalistic visual experiences [11]. While these results have not yet reached human trials, they suggest a trajectory toward functionally rich vision restoration.
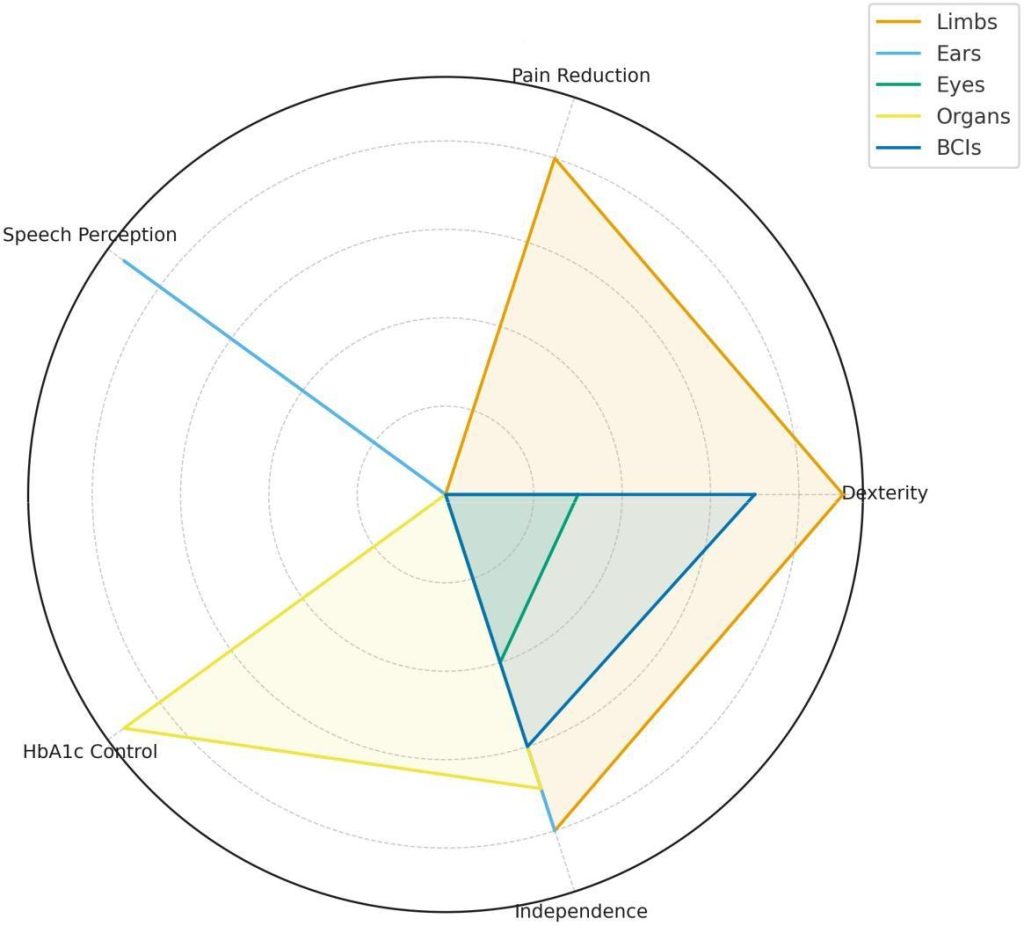
Fig. 8: Clinical outcome radar across device categories (illustrative 0–10 scales). Limb outcomes reflect neuromusculoskeletal prosthesis and closed-loop feedback studies [1], [3]; speech perception for ears from cochlear implant literature [22], [24]; HbA1c control from artificial pancreas trials [23]; independence and dexterity improvements from BCI work [13], [14].
C. Cochlear Implants and Auditory Prostheses
Cochlear implants are the most clinically established bionic devices, with decades of long-term outcome data. Studies consistently show that recipients achieve near-normal speech perception in quiet environments, with 80–90% of adult users able to understand conversational speech [22], [24]. Pediatric recipients implanted before the age of two can develop speech and language skills comparable to hearing peers, underscoring the importance of early intervention.Limitations remain in music perception and speech-in-noise environments. Fine structure processing algorithms have partially addressed these gaps, while emerging optogenetic cochlear implants offer the possibility of finer frequency resolution by stimulating auditory neurons with light rather than electricity [24]. Though still in early trials, such devices could overcome the channel interaction limits of electrode arrays.
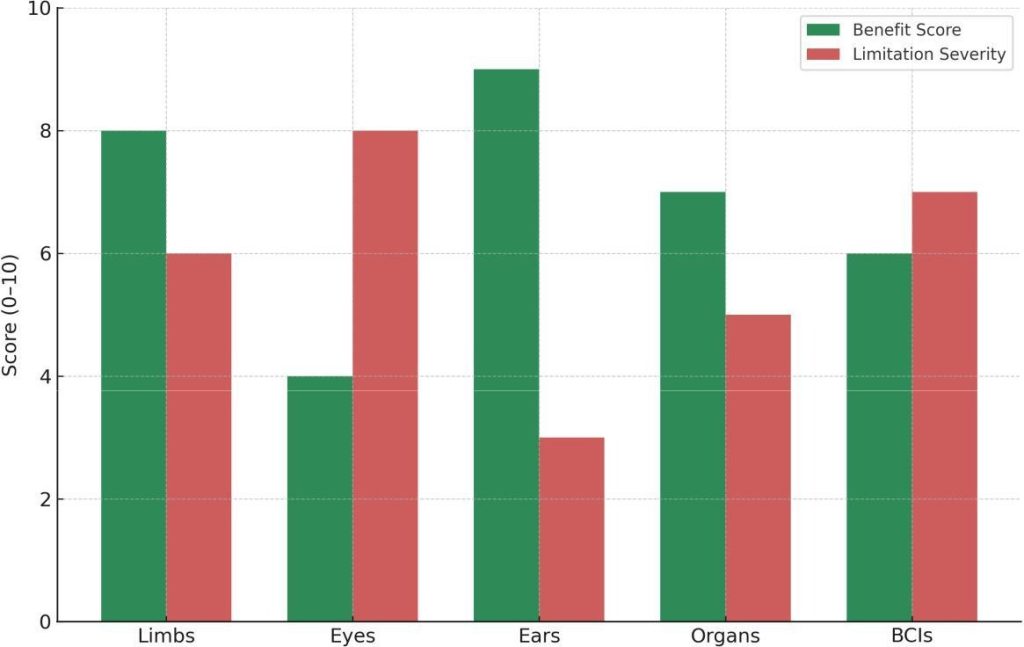
Fig. 9: Patient-centered benefits vs. limitation severity (illustrative 0–10). Scores are synthesized from clinical and review reports across limbs [1], [3], [15], eyes [5], [10], [11], ears [22], [24], organs [23], and BCIs [13], [14].
D. Artificial Pancreas
The artificial pancreas has advanced from inpatient feasibility studies to widespread outpatient use. Closed-loop systems integrating continuous glucose monitors and insulin pumps have demonstrated significant clinical benefits.
In the Cambridge Artificial Pancreas trials, adults and adolescents with type 1 diabetes using closed-loop control spent over 70% of the day in target glucose range, compared to about 50% with conventional therapy [23]. HbA1c levels improved by approximately 0.5%, and hypoglycemia episodes were reduced by more than 40%. These results have led to regulatory approval of hybrid closed-loop systems (Medtronic 670G, Tandem Control-IQ).
Beyond diabetes, closed-loop bioelectronic devices for hypertension and epilepsy are under investigation, suggesting that the artificial pancreas is a prototype for a broader class of organ-level bionics.
E. Brain–Computer Interfaces in Clinical Translation
BCIs are increasingly being tested in patients with paralysis. Hochberg et al. demonstrated that tetraplegic individuals could use cortical implants to control robotic arms with multiple degrees of freedom, achieving self-feeding tasks [14]. More recently, bidirectional BCIs delivering somatosensory feedback via cortical stimulation have restored not only motor intent but also tactile perception in paralyzed patients [13].
Non-invasive BCIs, while less precise, have enabled basic communication in locked-in syndrome. EEG-based spellers, though slow, offer a lifeline for individuals otherwise unable to interact with their environment. Clinical usability, however, remains limited by low bandwidth and the need for expert calibration.
TABLE V: Summary of Clinical Outcomes in Major Bionic Devices
| Device / System | Clinical Outcomes | Patient-Reported Impact | Limitations |
| Neuromusculoskeletalarm [1] | Improved SHAP score(+23%); reducedphantom pain | Daily wear; reduceddisability | Surgical risk;infection |
| Touch + kinesthesiaprosthesis [3] | Natural visuomotorbehavior; improveddexterity | Prosthesis ownership;intuitive use | Requires surgicalreinnervation |
| Microprocessorknees [6] | Improved gaitsymmetry; fewer falls | Higher confidence;mobility | Cost; battery life |
| Osseointegratedprosthesis [15] | Increased walkingendurance; comfort | Longer wear times;better stability | Infection risk |
| Argus II retinalimplant [5] | Light detection; objectlocalization | Independence innavigation | Low resolution |
| Perovskite/organicretinas [10], [11] | Preclinical visionrestoration; color vision | Potential fornaturalistic vision | Not yet in clinicaltrials |
| Cochlearimplant [22], [24] | Near-normal speech inquiet | Major quality of lifeimprovement | Music and noiselimitations |
| Artificialpancreas [23] | HbA1c reduction; >70%time in range | Reduced cognitiveburden | Device cost;calibration |
| Cortical BCI [13],[14] | Multi-DoF roboticcontrol; sensoryrestoration | Restoredindependence intasks | Invasive surgery;stability issues |
F. Patient Perspectives and Usability Studies
Across device types, patient-reported outcomes highlight both the benefits and limitations of bionic devices:
- Users of osseointegrated prostheses report higher comfort and daily wear time but express concerns about infection risk [15].
- Cochlear implant recipients overwhelmingly report improved quality of life, but many remain dissatisfied with music enjoyment [24].
- Bionic limb users often emphasize the importance of intuitive control and sensory feedback, without which devices are often abandoned [3].
- Artificial pancreas users report reduced cognitive burden, as the system automates much of the constant decision-making in diabetes care [23].
VI. Challenges and Limitations
While recent years have witnessed transformative advances in bionic technology, clinical translation remains constrained by significant technical, biological, and regulatory challenges. These limitations underscore the gap between laboratory performance and long-term, real-world usability.
A. Technical Challenges
- Signal Reliability and Noise: Surface EMG, EEG, and even implanted electrodes are subject to signal drift, noise, and instability over time. Sweat, skin impedance, and electrode displacement degrade surface signals, while implanted electrodes may shift microscopically due to tissue remodeling. These issues lead to loss of calibration, forcing frequent retraining of pattern-recognition algorithms [3]. Reinforcement learning approaches partially address this, but stable long-term decoding remains elusive.
- Power Supply and Energy Efficiency: Most prosthetic systems rely on rechargeable lithium-ion batteries, which add weight and require frequent charging. High-resolution bionic eyes and neural stimulators demand significant power for continuous operation, yet miniaturized power systems remain limited. Energy harvesting from body motion, thermoelectric gradients, or biofuel cells has been explored but is not yet clinically viable.
- Durability and Mechanical Robustness: Devices implanted in the body must withstand years of mechanical stress. MEMS sensors, polymer electrodes, and microelectronics may degrade under physiological conditions. Similarly, osseointegrated implants must tolerate repetitive load-bearing without loosening. Failures not only compromise device function but may necessitate surgical revision, which carries additional risk [15].
- Limited Bandwidth of Neural Interfaces: Even state-of-the-art cortical arrays record from a few hundred neurons, far below the millions involved in natural motor control. Intraneural electrodes provide some selectivity but risk damaging nerve fascicles. As a result, current devices cannot yet replicate the information throughput of the intact nervous system, restricting fine dexterity and natural sensory richness [1], [3].
B. Biological and Clinical Challenges
- Immune Response and Biocompatibility: Foreign-body response to implanted electrodes leads to fibrotic encapsulation, increasing impedance and reducing signal quality over time. Flexible polymers (polyimide, parylene-C) mitigate this mismatch, but chronic inflammation remains a barrier to decades-long stability [9]. Similarly, neural tissue is highly sensitive; intraneural arrays risk long-term axonal degeneration.
- Infection Risk in Osseointegration: Osseointegrated prostheses solve socket issues but leave a skin breach vulnerable to infection, despite titanium’s biocompatibility and antimicrobial coatings [15]. For many surgeons, this is the key clinical barrier to adoption.
- User Variability: Each patient presents unique residual anatomy, nerve distribution, and physiology. This heterogeneity makes one-size-fits-all solutions impractical. A prosthesis calibrated for one individual may fail in another with different EMG signal distribution. Personalized adaptation through AI offers promise but requires extensive data and user training [12].
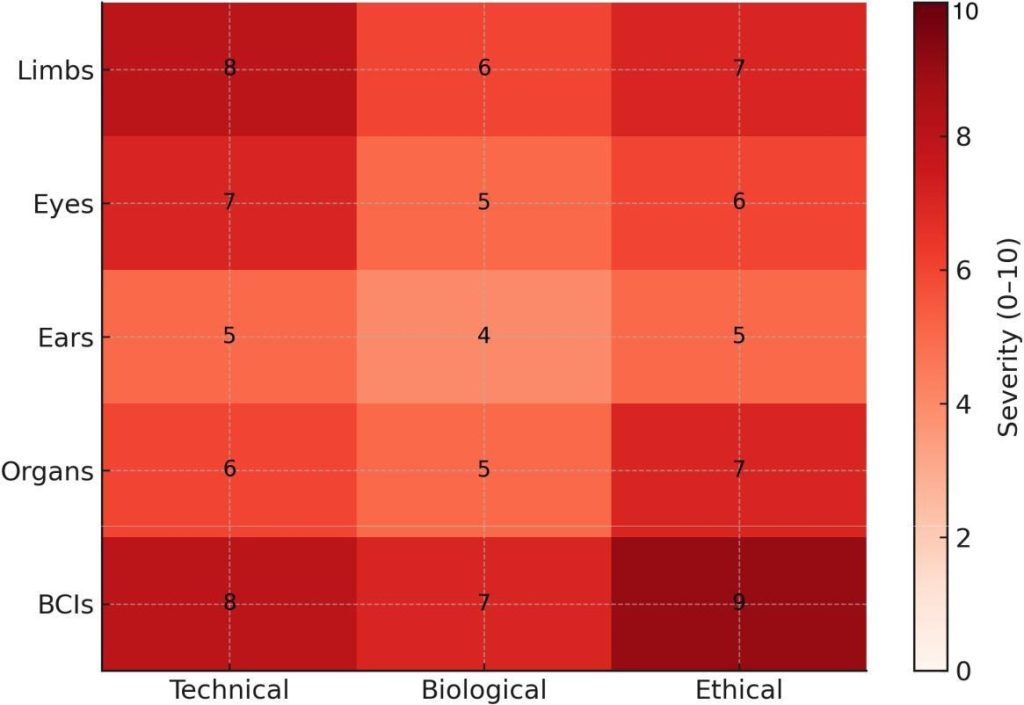
Fig. 10: Challenge severity heatmap (0–10) across technical, biological, and ethical domains. Entries are informed by clinical/engineering overviews [9], osseointegration cohorts [15], device and interface reports [1], [3], and neurotechnology ethics guidance [25].
- Rehabilitation and Training Burden: Complex bionic devices demand intensive rehabilitation. For example, users of targeted reinnervation prostheses must undergo weeks of training to learn new muscle–nerve mappings [3]. Without structured rehabilitation, even advanced devices risk abandonment. Successful adoption also depends on continuous feedback from clinicians, adaptive training software, and strong patient motivation. Limited access to rehabilitation resources further compounds these challenges, highlighting the need for more intuitive control strategies and scalable support systems.
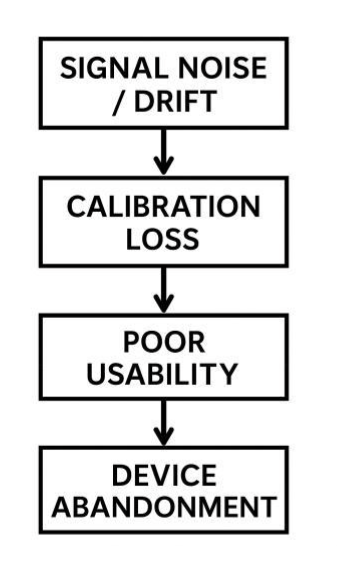
Fig. 11: Failure pathway flowchart: signal noise/drift → calibration loss → poor usability → device abandonment.
C. Ethical and Regulatory Challenges
- Accessibility and Equity: Advanced prostheses such as neuromusculoskeletal arms or retinal implants cost tens of thousands of dollars, often exceeding insurance coverage. As a result, access is restricted to high-resource healthcare systems, leaving a vast majority of potential users worldwide without benefit.
- Privacy and Data Security: Brain–computer interfaces generate continuous neural data streams, raising concerns about privacy, surveillance, and misuse. Questions of ownership and protection of neural data are critical as BCIs move toward commercial use [25].
- Regulatory Uncertainty: Regulatory frameworks (FDA, EMA) struggle to classify hybrid devices that combine hardware, software, and surgical procedures. Is a neuromusculoskeletal prosthesis a medical device, an implant, or a drug–device combination? These uncertainties slow approval processes and complicate clinical translation.
- Psychological and Social Factors: Bionic devices affect not only physiology but also identity. While many users embrace prostheses as part of their body schema, others experience alienation. High expectations, fueled by media portrayals of “superhuman cyborgs,” may lead to disappointment when real devices fall short. Social stigma and lack of support also contribute to device abandonment [17].
TABLE VI: Challenges and Limitations of Bionic Devices Across Categories
| Device Category | Technical Challenge | BiologicalChallenge | Ethical/RegulatoryChallenge |
| Bionic Limbs | Signal drift; limitedbandwidth | Infection risk(osseointegration) | High cost; limitedaccessibility |
| Bionic Eyes | Low resolution; highpower use | Retinal scarring(implants) | Limited approvalpathways |
| CochlearImplants | Limited frequencyresolution | Variable outcomes inlate-deafened users | Access inlow-incomecountries |
| ArtificialPancreas | Sensor lag; pumpprecision | Skin reactions tosensors | Insurance coverage;affordability |
| Brain–ComputerInterfaces | Low neuronsampling; instability | Neural tissuedamage;encapsulation | Privacy of neuraldata; unclearregulation |
D. Toward Overcoming Limitations
Efforts to address these limitations include:
- Flexible bioelectronics that minimize immune response [11].
- Antimicrobial and regenerative coatings for osseointegration [15].
- On-device AI that adapts to signal drift in real time [12].
- Energy harvesting systems that exploit body heat or motion [20].
- Ethical frameworks for neural data protection being drafted by international bioethics committees [25].
While none of these solutions is definitive, the rapid pace of interdisciplinary research suggests that many current barriers may be partially overcome within the next decade.
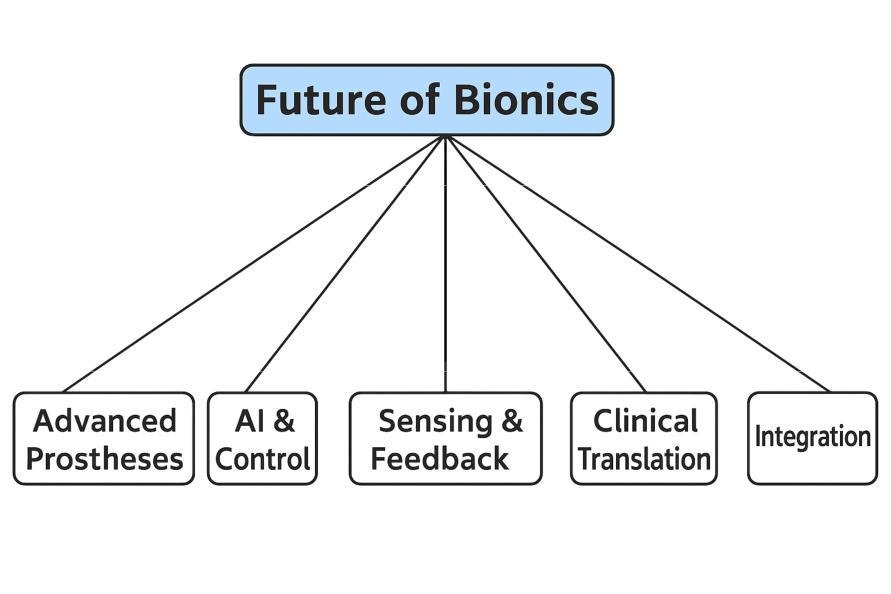
Fig. 12: Conceptual framework for the Future of Bionics. The figure highlights five major domains expected to shape ongoing advances: Advanced Prostheses, AI & Control, Sensing & Feedback, Clinical Translation, and Integration.
VII. Future Directions
The future of bionic devices lies in the pursuit of seamless human–machine integration, where artificial systems are no longer merely tools but functional extensions of the body. This vision depends on advances across materials science, neuroscience, artificial intelligence, and clinical medicine. The following subsections outline key directions for research and development.
A. Closed-Loop Systems
One of the most transformative frontiers in bionics is the realization of closed-loop systems that integrate sensing, computation, and actuation in a continuous feedback cycle. Current devices often operate in open-loop: users send motor commands, but feedback is limited or absent. This mismatch increases cognitive burden and reduces naturalism.
- Closed-Loop Limb Prostheses: Future prostheses will combine multimodal sensory feedback (touch, proprioception, temperature) with real-time motor decoding. For example, Ortiz-Catalan’s osseointegrated system demonstrates that stable long-term recording and stimulation are possible [1]. The next step is to expand sensory channels, potentially incorporating thermal sensors like those proposed in thermally sentient limb prototypes [8].
- Closed-Loop Vision: Neuromorphic retinas already integrate preprocessing [10], [11]. Coupled with cortical implants capable of delivering spatially distributed stimulation, future bionic eyes may offer continuous adaptive vision, adjusting to light conditions, motion, and attention demands.
- Closed-Loop Organ Devices: The artificial pancreas exemplifies the power of closed-loop design, and similar architectures may be applied to renal replacement (bionic kidney) or cardiac regulation (bioelectronic pacemakers with adaptive control). These devices would monitor physiological parameters continuously and autonomously adjust output, minimizing user intervention.
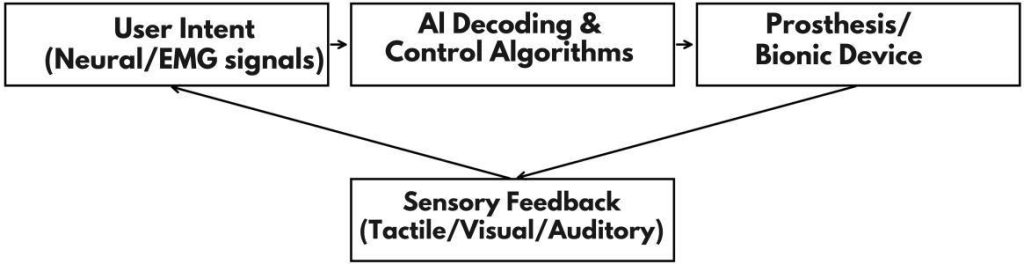
Fig. 13: AI-driven closed loop in bionic devices: user intent → AI decoding/control → device actuation; multimodal sensory feedback closes the loop and adapts the user.
B. Artificial Intelligence Integration
AI is expected to play a central role in the next generation of bionics, from signal decoding to personalized adaptation.
- Adaptive Decoding: Deep neural networks can outperform traditional pattern recognition in EMG/EEG classification, particularly under noisy conditions. Reinforcement learning approaches demonstrate that systems can learn alongside users, adapting to signal drift and improving control fidelity without explicit recalibration [12].
- Personalization: AI enables prostheses to learn user-specific patterns, such as grip preferences, walking dynamics, or habitual tasks. Over time, the device can anticipate actions, moving toward predictive control rather than reactive operation.
- AI in Sensory Processing: In vision and hearing prostheses, AI can denoise input signals, highlight salient features, or adapt stimulation patterns for optimal perception. For example, a bionic eye might enhance contrast in low-light conditions or suppress irrelevant motion, mimicking biological attentional mechanisms.
C. Fully Implantable Energy Systems
Energy supply remains a critical barrier. A major future direction is the development of fully implantable, autonomous power sources.
- Biofuel Cells: Glucose biofuel cells convert glucose and oxygen from bodily fluids into electricity. Early prototypes have powered pacemakers in animal models, suggesting feasibility for low-power implants.
- Energy Harvesting: Thermoelectric generators can exploit the temperature gradient between body heat and ambient air, while piezoelectric harvesters generate power from motion. If scaled effectively, such technologies could eliminate the need for external charging.
- Wireless Power Transfer: Mid-field and ultrasound-based wireless charging systems are being explored as alternatives to inductive coupling, enabling deeper implants to be recharged without bulky external coils.
D. Advanced Materials and Interfaces
- Living Electrodes: Incorporating stem-cell–derived neurons into electrode arrays could reduce immune rejection and improve signal fidelity.
- Self-Healing Polymers: Materials capable of repairing microcracks would extend device longevity under mechanical stress.
TABLE VII: Emerging Research Trends and Future Directions in Bionics
| Domain | Emerging Innovation | Anticipated Impact(5-10 years) | Example Studies | |
| Limb Prostheses | Bidirectional,multimodal feedback | Naturalistic control andembodiment | Ortiz-Catalan(2023) [1], Marasco(2021) [3] | |
| Vision | Neuromorphic retinas | Color, wide-FoVartificial vision | Long (2023) [10],Zhang (2023) [11] | |
| Hearing | Optogenetic cochlearimplants | Finer frequencyresolution; musicperception | Wilson (2017) [24] | |
| Organ Bionics | Dual-hormone artificialpancreas | Near-physiologicalglycemic control | Hovorka (2011) [23] | |
| Energy | Glucose biofuel cells;wireless charging | Fully implantableautonomous power | Nat. Commun.(2024) [18] | |
| NeuralInterfaces | Wireless minimallyinvasive arrays | Home-use BCIs forparalysis | Hochberg(2012) [14] | |
- Nanostructured Coatings: Surfaces engineered at the nanoscale can reduce bacterial adhesion (limiting infection risk) while promoting neural growth.
E. Ethical, Social, and Regulatory Horizons
As devices become more integrated and powerful, ethical considerations will intensify.
- Cognitive Autonomy: Closed-loop BCIs blur the boundary between user intent and machine response, raising questions about responsibility and agency.
- Human Enhancement vs. Therapy: While most bionics are designed for rehabilitation, the same technologies could be repurposed for augmentation, e.g., enhanced vision beyond the human spectrum.
- Global Accessibility: Future efforts must prioritize equitable distribution, ensuring that breakthroughs benefit not only high-income nations but also the global disabled population.
F. A 5–10 Year Outlook
Within the next decade, it is realistic to expect:
- Commercially available bidirectional prostheses with tactile and kinesthetic feedback.
- Next-generation artificial pancreas systems incorporating dual-hormone control (insulin + glucagon) for near-normal glycemic regulation.
- Retinal prostheses with color vision based on neuromorphic phototransistor arrays.
- BCIs with wireless, minimally invasive arrays enabling everyday use in home environments.
- Early adoption of autonomous power systems, reducing dependence on external charging.
In parallel, advances in AI, regenerative medicine, and material science will continue to converge, driving bionics toward devices that are smaller, smarter, and more biologically integrated.
Fig. 14: Convergence of disciplines: neuroscience, engineering, AI, and ethics. The overlap represents future bionics as seamless human–machine symbiosis.
G. Toward Convergence of Disciplines
The future of bionics will not be driven by any single technology but by the convergence of multiple fields. Neuroscientists, engineers, material scientists, ethicists, and clinicians must collaborate closely to translate prototypes into scalable, safe, and accessible devices. If this trajectory is sustained, the coming decades may witness the realization of functional, lifelong, fully integrated artificial organs and limbs, transforming rehabilitation and human–machine symbiosis.
VIII. Conclusion
Bionic devices have evolved from crude mechanical substitutes to sophisticated systems capable of bidirectional integration with the human nervous system. The landscape now includes neuromusculoskeletal limb prostheses, neuromorphic bionic eyes, cochlear implants, artificial pancreas systems, and experimental brain–computer interfaces. Across these categories, the central trend is clear: modern bionics aim not merely to restore function but to recreate the natural sensory–motor loop, thereby enhancing embodiment, autonomy, and quality of life.
Key technological enablers include biocompatible materials (e.g., titanium for osseointegration, organic semiconductors for neuromorphic sensors), advanced sensors and actuators (MEMS tactile arrays, series elastic actuators), and high-bandwidth neural interfaces (intraneural electrodes, hybrid nerve constructs).
Artificial intelligence and machine learning are now integral, allowing devices to adapt dynamically to user variability and environmental change.
Clinical studies demonstrate tangible benefits: improved dexterity, reduced phantom pain, restored sensory perception, and automated metabolic regulation. Yet significant challenges remain. Technical barriers such as signal instability and power supply, biological risks including immune response and infection, and ethical concerns over equity and neural data privacy all impede widespread adoption.
Looking forward, the field is poised for breakthroughs in closed-loop systems, AI-driven personalization, fully implantable energy solutions, and multimodal sensory feedback. Within the next decade, it is realistic to envision prostheses that feel like natural limbs, artificial organs that self-regulate without intervention, and BCIs that allow paralyzed individuals to regain independence in daily life.
Ultimately, the future of bionics lies in the convergence of disciplines—neuroscience, engineering, medicine, and ethics—to create devices that are not only technologically advanced but also safe, equitable, and meaningful for users. The vision of lifelong, seamlessly integrated artificial organs and limbs is no longer science fiction, but an achievable milestone within a generation.
References
- M. Ortiz-Catalan et al., “A highly integrated bionic hand with neural control and feedback for use in daily life,” Sci. Robot., vol. 8, no. 77, eadf7360, 2023.
- S. Dosen, “Toward self-contained bidirectional bionic limbs,” Sci. Robot., vol. 8, no. 77, eadk6086, 2023.
- S. Marasco et al., “Neurorobotic fusion of prosthetic touch, kinesthesia, and movement,” Sci. Robot., vol. 6, no. 59, eabf3368, 2021.
- C. Pasluosta et al., “Bidirectional bionic limbs,” J. Neural Eng., vol. 19, no. 1, 013001, 2022.
- U.S. Food and Drug Administration, “Argus II Retinal Prosthesis System—Summary of Safety and Effectiveness Data,” FDA, 2011.
- T. R. Clites et al., “Design and clinical implementation of an open-source bionic leg,” Nat. Biomed. Eng., vol. 4, pp. 941–952, 2020.
- Y. Cho et al., “Hybrid bionic nerve interface for application in bionic limbs,” Adv. Sci., vol. 10, no. 5, 2206859, 2023.
- M. Ortiz-Catalan, “Thermally sentient bionic limbs,” Nat. Biomed. Eng., 2024.
- Editorial, “Advances in clinical and prosthetic care,” Front. Rehabil. Sci., vol. 3, 2022.
- Z. Long et al., “A neuromorphic bionic eye with filter-free color vision using hemispherical perovskite nanowire array retina,” Nat. Commun., vol. 14, 37581, 2023.
- H. Zhang et al., “A neuromorphic bionic eye with broadband vision and biocompatibility using TIPS-pentacene phototransistor array retina,” Appl. Mater. Today, vol. 32, 2023.
- H. R. Schone et al., “Biomimetic versus arbitrary motor control strategies for bionic hand skill learning,” Nat. Hum. Behav., vol. 8, pp. 1108–1123, 2024.
- S. N. Flesher et al., “Restored tactile sensation improves neuroprosthetic arm control,” Sci. Transl. Med., vol. 13, no. 612, 2021.
- L. R. Hochberg et al., “Reach and grasp by people with tetraplegia using a neurally controlled robotic arm,” Nature, vol. 485, pp. 372–375, 2012.
- R. Brånemark et al., “Osseointegrated percutaneous prostheses for patients with limb loss,” Bone Joint J., vol. 101-B, pp. 55–63, 2019.
- C. Pasluosta, P. Kiele, S. Micera et al., “The current state of bionic limbs from the surgeon’s viewpoint,” J. Neural Eng., vol. 19, no. 1, 2022.
- “The future of bionic limbs,” Prosthet. Orthot. Int., vol. 45, no. 5, 2021.
- “Clinical implementation of advanced bionic prostheses,” Nat. Commun., vol. 15, 2024.
- H. R. Schone et al., “Should bionic limb control mimic the human body? Impact of control strategy on bionic hand skill learning,” bioRxiv preprint, 2023.
- “Advances in prosthetic rehabilitation sciences,” Front. Rehabil. Sci., vol. 3, 2022.
- World Health Organization, Global Report on Rehabilitation, Geneva, 2022.
- P. C. Loizou, “Cochlear implants: Historical perspective and current applications,” IEEE Eng. Med. Biol. Mag., vol. 25, no. 5, pp. 40–46, 2006.
- L. Hovorka, “Closed-loop insulin delivery: From bench to clinical practice,” Nat. Rev. Endocrinol., vol. 7, pp. 385–395, 2011.
- B. C. Wilson, “The future of cochlear implants,” J. Assoc. Res. Otolaryngol., vol. 18, pp. 695–704, 2017.
- UNESCO International Bioethics Committee, “Ethical issues of neurotechnology,” Policy Report, 2021.
About the author

Vyomesh Vikram Singh
Vyomesh Vikram Singh is a recent high school graduate with interests in computer science, robotics, and edge AI. His projects have included automation systems, gesture-controlled vehicles, unmanned aerial platforms, and a low-cost Raspberry Pi projector designed for rural classrooms to help underprivileged children connect to the internet. He has also worked on model optimisation for IoT devices and authored a review on human–computer interfaces, bionic limbs, and neuromorphic vision.
His research interests include robotics, human–machine interaction, and efficient AI deployment on embedded systems. Beyond academics, Vyomesh also served as a board member of Adlers, the Photography Club. Combining technology with art, he applied machine learning to make his filmography distinct and innovative, earning recognition at multiple competitions, including 3rd prize in an international geography documentary competition in Geofest International. Vyomesh has contributed to leadership and creative pursuits including serving as the head of his school’s Robotics Club for 3 years, guiding peers in building prototypes and organising seminar series by inviting respected external speakers. He is also an intermediate guitar player, and tries to make people around him happy by his talent.
