
Author: Emerson G. Brown
Mentor: Dr. Adam Behensky
Lake Forest High School
Abstract
Chronic pain is a multifaceted condition characterized by persistent sensory, cognitive, and emotional distress that extends beyond normal healing time. Affecting nearly one in five individuals globally, chronic pain represents one of the leading causes of disability and reduced quality of life. Opioid analgesics, historically the cornerstone of moderate-to-severe pain treatment, are increasingly recognized as problematic in long-term use due to tolerance, dependence, hyperalgesia, and addiction risk. Consequently, there is an urgent need to identify alternative approaches that are both effective and sustainable. Recent scientific interest has turned to classic serotonergic psychedelics such as psilocybin, lysergic acid diethylamide (LSD), and N,N-dimethyltryptamine (DMT), which may exert analgesic effects through modulation of serotonin receptors, enhancement of neuroplasticity, and reorganization of large-scale brain networks involved in pain perception and emotional distress. This review synthesizes current knowledge of chronic pain neurobiology, evaluates the mechanisms and limitations of opioids, and examines emerging evidence for psychedelics in pain management. Particular focus is placed on receptor-level pharmacology, network-level brain circuit changes, and clinical evidence across pain disorders. While preliminary data suggest that psychedelics may offer durable relief and address both sensory and affective dimensions of pain, challenges remain regarding safety, variability of response, and regulatory hurdles. Psychedelics represent a promising, though experimental, paradigm shift in chronic pain management that warrants rigorous investigation.
Introduction
Chronic pain is not merely a prolonged form of acute pain but rather a distinct pathological state involving maladaptive changes in peripheral and central nervous system function [1,2]. Unlike acute pain, which serves an adaptive role by signaling tissue injury, chronic pain often persists independent of nociceptive stimuli and is increasingly understood as a disorder of central processing and neural plasticity [3]. The personal, societal, and economic impact of chronic pain is profound, with high prevalence, reduced productivity, psychiatric comorbidities such as depression and anxiety, and substantial health care utilization [4,5].
Traditional treatments for chronic pain include nonsteroidal anti-inflammatory drugs (NSAIDs), anticonvulsants, antidepressants, and opioids [6]. Of these, opioids remain the most potent pharmacological tools available, particularly for acute and cancer-related pain [7]. However, in the context of chronic non-cancer pain, long-term opioid therapy has proven controversial due to limited functional benefit and high risks of tolerance, dependence, and addiction [8,9]. The ongoing opioid crisis underscores the dangers of widespread opioid prescribing and highlights the necessity of alternative strategies [10].
Parallel to the opioid story, psychedelics have re-emerged in biomedical research after decades of prohibition. Early investigations in the mid-20th century explored their potential in psychiatry and pain management before regulatory restrictions halted progress [11]. In recent years, renewed interest has focused on the ability of psychedelics to modulate brain networks, enhance neuroplasticity, and produce long-lasting therapeutic effects across psychiatric conditions [12-14]. These properties raise the possibility that psychedelics could also play a role in addressing chronic pain syndromes.
This review aims to provide a comprehensive synthesis of chronic pain neurobiology, the pharmacological basis and limitations of opioids, and the mechanistic and clinical rationale for psychedelic-assisted approaches to pain.
Chronic Pain and Its Neurobiology
Chronic pain is typically defined as pain that persists for longer than three months and extends beyond the expected period of tissue healing [15,16]. In contrast to acute pain—which is transient, protective, and usually linked to a discrete injury or illness—chronic pain represents a maladaptive condition characterized by persistent activation of pain pathways within both the peripheral and central nervous systems [3,4]. Rather than serving as a biological warning signal, chronic pain often becomes a disease state in itself, marked by complex neurobiological, psychological, and social dimensions [5].
The clinical manifestations of chronic pain are heterogeneous and can be categorized according to their underlying mechanisms and anatomical origins. Neuropathic pain arises from injury or dysfunction of the somatosensory nervous system and includes conditions such as peripheral neuropathy, sciatica, trigeminal neuralgia, and phantom limb pain, in which pain is perceived in an amputated limb [17]. Musculoskeletal pain involves bones, joints, and muscles, and is common in disorders such as osteoarthritis, rheumatoid arthritis, fibromyalgia, and myofascial pain syndrome [18]. Inflammatory pain develops in the context of immune dysregulation or persistent inflammation, as seen in autoimmune diseases such as systemic lupus erythematosus or localized conditions including tendinitis and bursitis [19]. Visceral pain, originating from internal organs, is often diffuse, difficult to localize, and exemplified by conditions such as irritable bowel syndrome, endometriosis, and chronic pelvic pain [20]. Finally, centralized pain is increasingly recognized as a distinct category, characterized by hypersensitivity of central nervous system processing mechanisms and evident in conditions such as fibromyalgia and chronic migraine. In these cases, pain persists without any ongoing peripheral injury and is frequently amplified by dysregulated central neural networks [4].
A key feature of chronic pain is its impact on brain circuitry. Neural processing of persistent pain involves the so-called “pain matrix, ” a distributed network of cortical and subcortical regions that underlies the sensory, affective, and cognitive dimensions of pain [21]. Within this matrix, the primary and secondary somatosensory cortices encode the intensity and spatial localization of painful stimuli, while the anterior cingulate cortex integrates the emotional salience of pain. The insula plays a central role in interoception and the integration of sensory information with autonomic and affective states, and the prefrontal cortex contributes to higher-order modulation, decision-making, and appraisal of pain experiences [22]. Neuroimaging studies have demonstrated that chronic pain is associated with structural and functional remodeling of these regions, including altered gray matter density, aberrant connectivity, and maladaptive patterns of activation [23].
These changes are underpinned by neuroplasticity, the brain’s capacity to reorganize and form new synaptic pathways in response to persistent stimuli. While neuroplasticity normally supports adaptation and learning, in the context of chronic pain it becomes maladaptive, leading to hyperexcitability of nociceptive pathways, enhanced pain perception, and decreased efficacy of endogenous pain inhibition [3]. Such changes can also contribute to psychological comorbidities, including depression, anxiety, and cognitive impairment, further compounding the burden of chronic pain. Thus, rather than being a passive symptom, chronic pain represents a dynamic driver of neurobiological remodeling that perpetuates its own persistence and complexity [5].
Opioid Treatment for Pain Management
Opioids have long been employed as a primary pharmacological intervention for a range of chronic pain conditions, yet their clinical efficacy and appropriateness depend critically on the underlying etiology and severity of pain [24]. Mild to moderate pain, such as early-stage musculoskeletal disorders or postoperative discomfort, is often managed with lower-potency opioids, including codeine or tramadol. While these agents are comparatively less potent than full agonists, they nonetheless carry notable risks of dependence and addiction. For moderate to severe pain, particularly neuropathic conditions such as diabetic neuropathy or sciatica, stronger opioids—such as morphine, oxycodone, or hydrocodone—are typically prescribed. In instances of severe, refractory pain, including advanced cancer or complex regional pain syndrome, high-potency opioids such as hydromorphone or fentanyl may be indicated. Fentanyl, in particular, exhibits up to 100-fold greater potency than morphine and is generally reserved for patients with significant opioid tolerance [24]. Notably, opioids demonstrate limited efficacy in certain centralized pain syndromes, including fibromyalgia, where non-opioid interventions are often preferred.
The analgesic effects of opioids are primarily mediated through the activation of mu-opioid receptors (μORs), a class of G-protein-coupled receptors distributed throughout both the central and peripheral nervous systems [25]. Within the brain, MORs are densely expressed in key regions associated with pain processing, modulation, and affective response, including the periaqueductal gray (PAG), thalamus, anterior cingulate cortex (ACC), amygdala, and nucleus accumbens (NAc). MOR activation inhibits adenylate cyclase activity, reduces cyclic AMP production, decreases calcium influx, and increases potassium efflux across neuronal membranes, leading to hyperpolarization and attenuated excitability of nociceptive neurons. In the PAG and rostral ventromedial medulla (RVM), opioids engage descending inhibitory pathways, while in the thalamus and somatosensory cortices, they modulate sensory-discriminative aspects of pain. Simultaneously, opioid effects in the amygdala and prefrontal cortex influence the emotional and cognitive dimensions of pain perception. Importantly, the NAc and ventral tegmental area (VTA) mediate opioid-induced reward and reinforcement, contributing to the high addictive potential of these drugs [25].
Chronic opioid exposure frequently results in tolerance, necessitating escalating doses to maintain analgesia, and can precipitate physical dependence and addiction. The activation of reward circuits further reinforces compulsive drug-seeking behaviors. Adverse effects such as respiratory depression, constipation, sedation, and endocrine dysregulation complicate long-term therapy and can significantly impair quality of life. Moreover, opioids often fail to adequately address the affective and psychological components of chronic pain, which are central to its persistence and functional impact. Collectively, these limitations underscore the pressing need for alternative pain management strategies that target both the sensory and affective dimensions of pain while minimizing the risks associated with chronic opioid therapy.
Psychedelics: An Emerging Alternative
Classic serotonergic psychedelics, including psilocybin, lysergic acid diethylamide (LSD), and N,N-dimethyltryptamine (DMT), have garnered increasing attention for their potential role in the management of chronic pain [26]. Both preclinical and clinical investigations suggest that these compounds may exert analgesic effects through mechanisms involving agonism of the serotonin 5-HT2A receptor and modulation of functional connectivity across key brain regions implicated in pain processing, including the thalamus, anterior cingulate cortex (ACC), and prefrontal cortex (PFC) [27]. Importantly, psychedelics appear to disrupt rigid neural patterns and entrenched circuit dynamics, a property that may be particularly relevant for chronic pain conditions in which affective, cognitive, and perceptual factors contribute substantially to symptom persistence [27].
Pain is inherently a subjective experience, shaped not only by peripheral nociceptive input but also by higher-order emotional, cognitive, and contextual influences. This complexity is exemplified by disorders such as fibromyalgia, which often lack identifiable tissue pathology and are considered central sensitization syndromes. In these conditions, patients frequently perceive pain as an emergent property of altered central processing rather than a direct reflection of peripheral injury [28]. Therapeutic interventions such as hypnosis and mindfulness-based practices have demonstrated that pain perception can be modulated by top-down processes, highlighting the brain’s capacity to suppress or reframe nociceptive experiences. Psychedelics may act through analogous mechanisms, promoting neuroplasticity and facilitating the decoupling of maladaptive neural circuits from conscious pain perception, thereby enabling patients to reinterpret or disengage from their pain experience [28].
Emerging evidence suggests that the analgesic effects of psychedelics can be durable. Clinical studies have reported that single or limited psychedelic-assisted sessions may produce sustained reductions in pain intensity lasting several weeks or months, in contrast to opioids, which require repeated administration and are associated with tolerance, dependence, and opioid-induced hyperalgesia [29]. These enduring effects are thought to arise from profound modulation of brain network dynamics, particularly within the default mode network (DMN), and enhanced integration of sensory, limbic, and prefrontal circuits, which collectively alter both the perception and the affective salience of pain [29].
Despite their promise, psychedelic therapies are not without limitations. Individual responses are highly variable, and while many patients report significant improvements in pain and quality of life, adverse psychological reactions—such as anxiety, paranoia, or perceptual disturbances—can occur [30]. Psychedelics are contraindicated in individuals with a history of psychosis or certain mood disorders, necessitating careful screening and administration within controlled therapeutic settings [30]. Furthermore, legal and regulatory barriers pose substantial challenges, as most classic psychedelics are classified as Schedule I substances under federal law, restricting clinical access and research opportunities [30].
In summary, serotonergic psychedelics represent a compelling, mechanistically distinct alternative to conventional pharmacologic therapies for chronic pain, particularly in conditions that are refractory to standard interventions. Their capacity to modulate both neurobiological circuits and cognitive-affective frameworks of pain positions them as a promising, though complex, frontier in pain medicine [26, 27,30]. Ongoing research is required to elucidate their precise mechanisms of action, optimize dosing protocols, and establish robust safety and efficacy profiles to support their responsible integration into clinical practice.
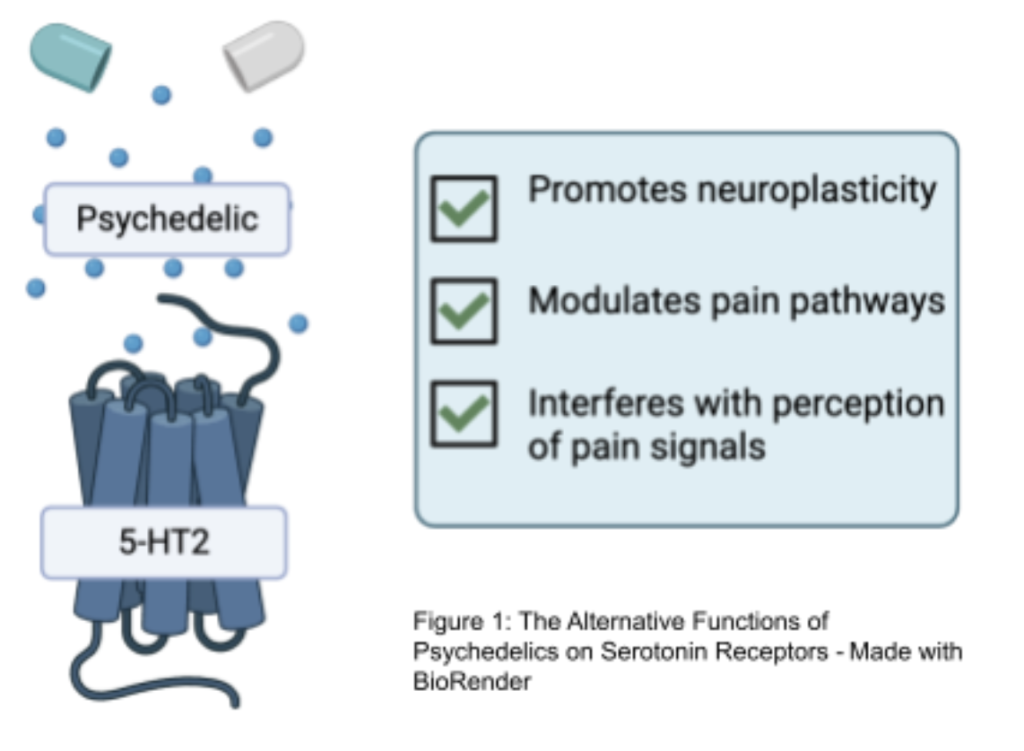
Serotonin Receptors and Psychedelic Action
The analgesic and therapeutic potential of classic psychedelics in chronic pain management is closely linked to their modulation of serotonin receptors, with the 5-HT2A receptor playing a central role, as seen in figure 2 [31]. These receptors are densely expressed in cortical regions critical for pain processing, including the prefrontal cortex (PFC), anterior cingulate cortex (ACC), and insula, which collectively govern sensory perception, cognitive evaluation, and affective integration of pain. 5-HT2A receptors are also present in subcortical structures such as the thalamus and amygdala, which mediate the relay of nociceptive signals and the emotional appraisal of painful stimuli [31].
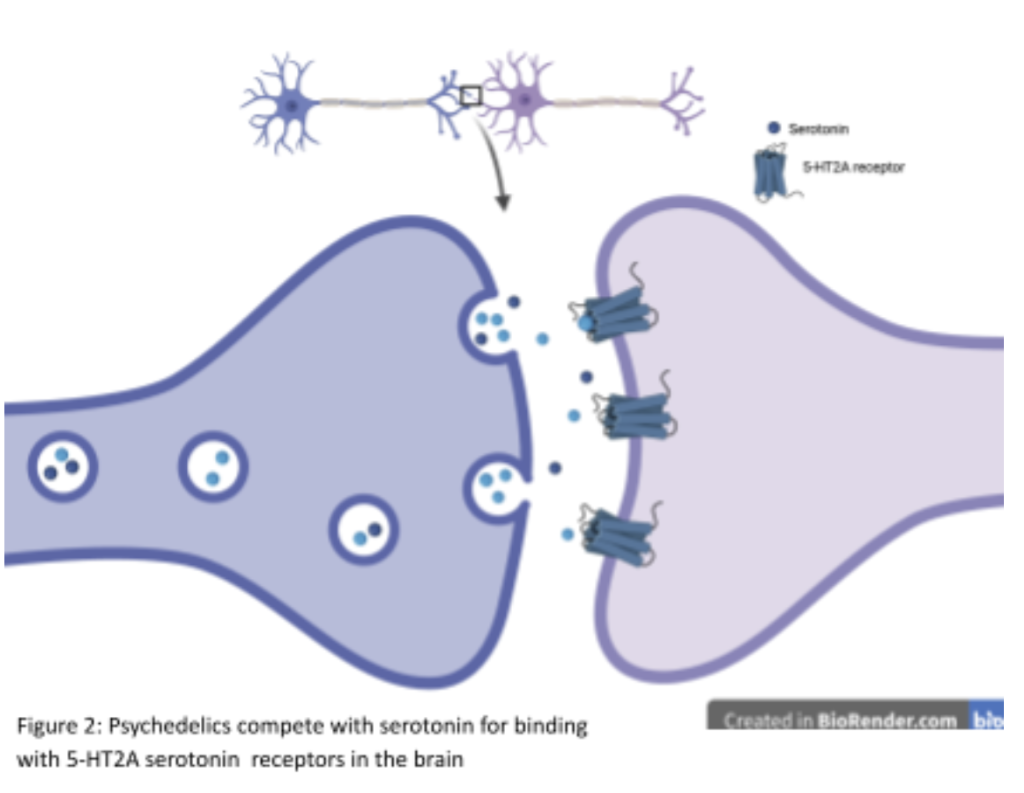
Activation of 5-HT2A receptors by serotonergic psychedelics, including psilocybin and LSD, enhances excitatory glutamatergic signaling within cortical pyramidal neurons [32]. This increase in cortical excitability facilitates greater inter-regional connectivity and disrupts rigid neural network dynamics, mechanisms thought to underpin both the altered states of consciousness characteristic of the psychedelic experience and the observed analgesic effects [32]. Functional neuroimaging in humans has demonstrated that psychedelic-induced 5-HT2A activation reduces coherence within the default mode network (DMN) while simultaneously enhancing connectivity between sensory, limbic, and associative cortical regions. These network-level changes correlate with reductions in perceived pain intensity and improvements in cognitive and emotional coping with pain [32].
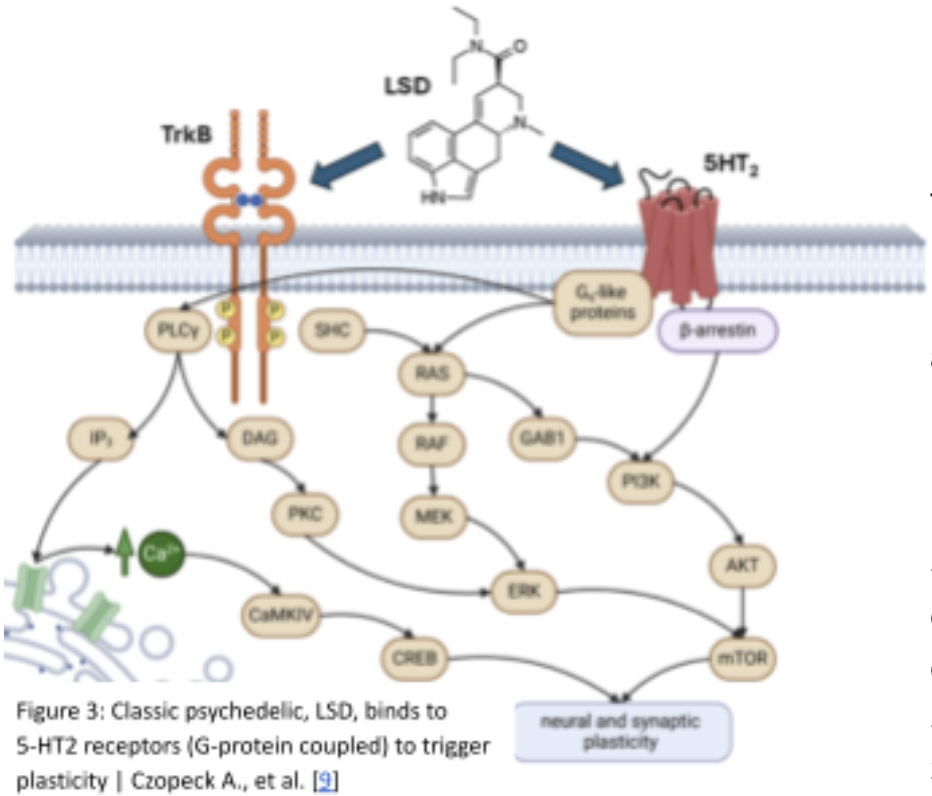
Preclinical studies further support the mechanistic role of 5-HT2A signaling in pain modulation. Animal models reveal that 5-HT2A agonists reduce nocifensive behaviors, whereas pharmacological blockade of these receptors abolishes the analgesic effects, underscoring a causal link between serotonergic modulation and pain attenuation [33]. Additional serotonin receptor subtypes, including 5-HT1A and 5-HT2C, may also contribute to anxiolytic effects and affective regulation, complementing the analgesic properties of 5-HT2A activation [33]. As seen in figure 3, these findings suggest that modulation of serotonin receptors—particularly 5-HT2A agonism—constitutes a key mechanistic bridge connecting psychedelic neuropharmacology to durable reductions in chronic pain [31-33].
Brain Circuits Linking Pain and Psychedelic Experience
Pain perception is mediated by a distributed neural network that integrates both sensory-discriminative and affective-motivational dimensions. The thalamus serves as a critical relay, transmitting nociceptive signals to the primary and secondary somatosensory cortices, which encode the location, intensity, and quality of painful stimuli. Concurrently, the anterior cingulate cortex (ACC) and insula process the emotional and motivational aspects of pain, linking the sensory experience to affective and autonomic responses. These regions form a tightly interconnected system: thalamic projections reach both the somatosensory cortices and the ACC, while the ACC and insula communicate with the amygdala and prefrontal cortex (PFC) to regulate the emotional salience and cognitive appraisal of nociceptive input. The medial PFC, in particular, plays a pivotal role in self-referential processing, evaluating pain in the context of one’s personal identity, and often amplifying distress when pain is perceived as a threat to the self [27].
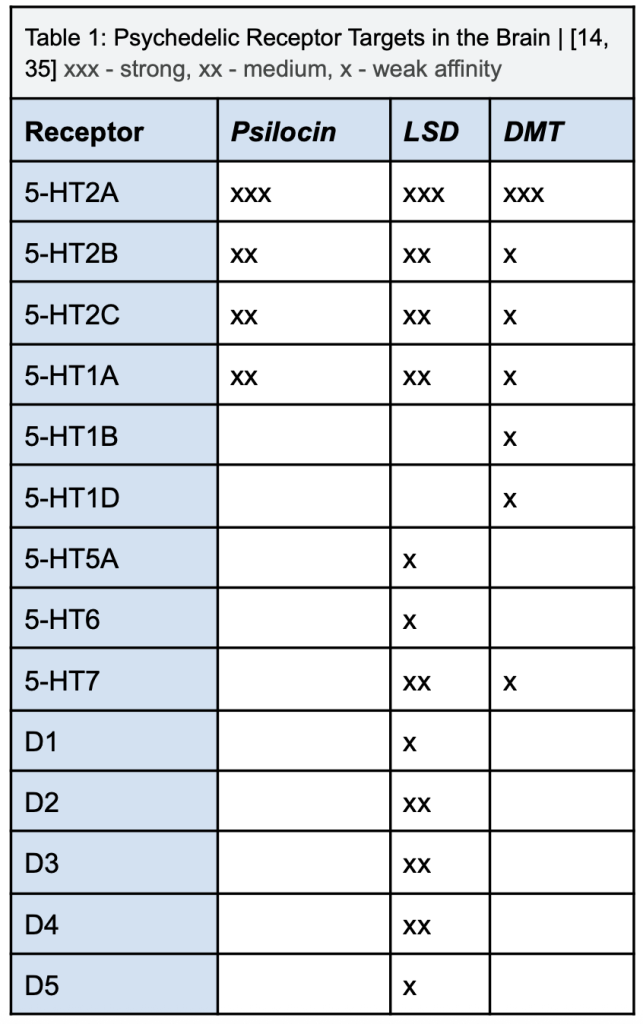
Classic serotonergic psychedelics, including psilocybin, LSD, and DMT, modulate these pain-related circuits by altering functional connectivity within and between the default mode network (DMN), limbic structures, and sensory pathways. As seen in table 1, their primary mechanism involves agonism at the 5-HT2A receptor, with additional contributions from 5-HT2C and 5-HT1A receptors [27]. Activation of 5-HT2A receptors enhances excitatory glutamatergic signaling in layer V cortical pyramidal neurons, promoting cross-talk between sensory and associative cortical regions while concurrently reducing top-down inhibitory control exerted by the DMN [27]. This disruption of rigid network dynamics leads to a disintegration of entrenched self-referential processing in the medial PFC and posterior cingulate cortex, effectively reshaping the emotional interpretation of pain. By weakening hyperconnected pain-related circuits—such as thalamus-ACC-PFC loops—and strengthening communication between limbic and sensory regions, psychedelics may attenuate the negative affective weight of nociceptive input and enhance cognitive flexibility in pain reappraisal [27].
Functional neuroimaging studies further demonstrate that psychedelics reduce DMN coherence and alter connectivity within the limbic system, including the amygdala and hippocampus, regions critically involved in the emotional amplification of pain [34]. Altered ACC and PFC activity under psychedelic influence has been associated with decreased affective suffering and improved adaptive coping strategies, while reductions in amygdala reactivity may diminish the emotional salience of pain. One emerging hypothesis posits that psychedelics “reset” maladaptive neural circuitry by promoting neuroplasticity and disrupting entrenched pain pathways, thereby enabling the reorganization of networks implicated in pain chronification [35]. This network “reset” may reduce hyperconnectivity within pain circuits and restore more adaptive patterns of brain activity, offering a novel neurobiologically informed avenue for chronic pain therapy [27,34].
Clinical Evidence and Trials
Recent studies provide preliminary evidence that classic psychedelics may modulate both the sensory-discriminative and affective-motivational dimensions of pain [36]. Early human research, including small pilot trials, has demonstrated reductions in headache burden and migraine frequency following psilocybin administration [33]. Complementary studies in healthy volunteers using crossover designs have reported increased tolerance to noxious stimuli, such as cold-pressor tasks, alongside reductions in the perceived unpleasantness of pain [36]. These effects are hypothesized to arise from 5-HT2A receptor-mediated reorganization of neural networks and top-down recalibration of salience and affective appraisal mechanisms [36].
Preclinical rodent models support these findings, showing that serotonergic psychedelics reduce inflammatory hyperalgesia and neuropathic allodynia while promoting cortical plasticity within thalamo-cortico-limbic circuits implicated in the chronification of pain [36]. Despite these encouraging results, current evidence is limited by several methodological constraints, including small sample sizes, open-label designs susceptible to expectancy effects, heterogeneous pain phenotypes, variable dosing regimens, limited active-placebo controls, short follow-up periods, and the frequent exclusion of individuals on long-term opioid therapy [36]. These factors constrain both generalizability and mechanistic inference [36].
Future research directions aim to overcome these limitations through rigorous, translationally oriented studies. Promising strategies include adequately powered randomized controlled trials with active placebo comparators, integration of quantitative sensory testing and neuroimaging (including resting-state and pain-evoked fMRI/EEG) to assess circuit-level changes, and the incorporation of inflammatory and neurotrophic biomarkers as potential mediators [33]. Comparative studies evaluating macrodosing versus low-dose protocols, the development of non-hallucinogenic 5-HT2A-biased analogs, and protocols that combine psychedelic administration with behavioral pain-rehabilitation or affect regulation training may further leverage experience-dependent neuroplasticity to optimize therapeutic outcomes [36].
Ethical, Regulatory, and Translational Challenges
The reintroduction of psychedelics into medicine raises ethical and regulatory challenges. Despite growing evidence, psychedelics remain Schedule I substances under international law, classified as having high abuse potential and no accepted medical use [37]. This status imposes significant barriers to research, including restricted funding and complex regulatory approvals [37].
Ethical concerns include the intensity and unpredictability of psychedelic experiences, the necessity of careful screening and psychotherapeutic support, and the potential for adverse psychological reactions [36]. Unlike opioids, psychedelics are not drugs of daily administration but are instead delivered in structured, supervised sessions. This model requires new infrastructure, trained facilitators, and integration with psychological care [35].
There are also questions of accessibility, cultural sensitivity, and equitable distribution. Without careful regulation, there is risk that psychedelic therapies could become commodified and inaccessible to those most in need [37].
Discussion
The emerging evidence comparing classic psychedelics to traditional opioid analgesics highlights fundamentally distinct mechanisms of action and clinical profiles. Opioids exert analgesia primarily through activation of G-protein-coupled mu-opioid receptors, leading to neuronal hyperpolarization and reduced nociceptive transmission [38]. While opioids are highly effective for acute pain, their long-term use is limited by tolerance, physical dependence, risk of misuse, and numerous adverse effects [39]. By contrast, classic serotonergic psychedelics act primarily as 5-HT2A receptor agonists and modulate pain indirectly by altering cortical network dynamics, affective processing, and cognitive appraisal rather than directly blocking nociceptive input [40]. This mechanistic distinction suggests that psychedelics may be particularly well-suited to chronic pain states with pronounced affective-cognitive components, such as neuropathic pain, headache disorders, and centralized pain syndromes [40].
Clinical evidence for psychedelic-assisted pain therapy is promising but remains preliminary. Systematic reviews, small controlled trials, and case series indicate that psychedelic interventions can reduce pain intensity, improve mood, and enhance functional outcomes [41]. However, sample sizes are small, study designs are heterogeneous, and large-scale randomized trials are largely lacking [41]. By comparison, opioids have robust evidence for short-term analgesia but demonstrate limited efficacy for many chronic pain conditions in the long term and carry well-documented risks [42].
Mechanistically, psychedelics appear to “reset” maladaptive neural circuits involved in pain chronification, promoting neuroplasticity and network reorganization that may reduce hyperconnectivity in pain-related pathways while enhancing adaptive processing of sensory and affective input [44]. This contrasts with opioids, which primarily suppress nociceptive signaling without addressing the cognitive and emotional dimensions of chronic pain [42]. Such network-level effects may allow psychedelics to provide sustained analgesia and improvements in quality of life following a limited number of treatment sessions, unlike opioids, which require ongoing administration and are associated with tolerance and dependence [41,42].
It is important to note that this review does not include non-classical psychoactive compounds such as ketamine, Salvia divinorum, or MDMA, each of which has distinct pharmacological and clinical profiles. Ketamine acts primarily as an NMDA receptor antagonist with rapid antidepressant and analgesic effects and interacts with the endogenous opioid system [40], whereas Salvinorin A, the active component of Salvia divinorum, is a potent kappa-opioid receptor agonist with dysphoric subjective effects [42]. MDMA is an entactogen whose primary actions involve robust monoaminergic release [41]. Furthermore, MDMA, Salvia divinorum, and ketamine are highly addictive drugs. Since these drugs do not overcome the risk of addiction, they are not a viable substitute for opioid in treating pain. These differences underscore that the current focus on classic serotonergic psychedelics provides a coherent mechanistic framework distinct from other psychoactive therapies [42].
Conclusion
In conclusion, classic psychedelics offer a mechanistically unique, non-opioid approach to chronic pain management, targeting both neurobiological and psychological dimensions. While preliminary findings suggest potential for durable analgesia, affective modulation, and network-level neuroplasticity, robust, adequately powered clinical trials and mechanistic studies are required. Careful attention to safety, therapeutic context, and regulatory constraints will be essential for translating these promising compounds into responsible clinical interventions that may complement or, in selected cases, provide alternatives to conventional opioid therapy.
References
1. Apkarian, A. V., Hashmi, J. A., & Baliki, M. N. (2011). Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain, 152(3 Suppl), S49–S64. https://doi.org/10.1016/j.pain.2010.11.010
2.Mills, S. E. E., Nicolson, K. P., & Smith, B. H. (2019). Chronic pain: a review of its epidemiology and associated factors in population-based studies. British journal of anaesthesia, 123(2), e273–e283. https://doi.org/10.1016/j.bja.2019.03.023
3. Drewes, A. M., Jensen, R. D., Nielsen, L. M., Droney, J., Christrup, L. L., Arendt-Nielsen, L., Riley, J., & Dahan, A. (2013). Differences between opioids: pharmacological, experimental, clinical and economical perspectives. British journal of clinical pharmacology, 75(1), 60–78. https://doi.org/10.1111/j.1365-2125.2012.04317.x
4. Carhart-Harris, R. L., & Friston, K. J. (2019). REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacological reviews, 71(3), 316–344. https://doi.org/10.1124/pr.118.017160
5. Dahlhamer, J., Lucas, J., Zelaya, C., Nahin, R., Mackey, S., DeBar, L., Kerns, R., Von Korff, M., Porter, L., & Helmick, C. (2018). Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults – United States, 2016. MMWR. Morbidity and mortality weekly report, 67(36), 1001–1006. https://doi.org/10.15585/mmwr.mm6736a2
6. Denk, F., McMahon, S. B., & Tracey, I. (2014). Pain vulnerability: a neurobiological perspective. Nature neuroscience, 17(2), 192–200. https://doi.org/10.1038/nn.3628
7. Mian, M. U., Afzal, M., Butt, A. A., Ijaz, M., Khalil, K., Abbasi, M., Fatima, M., Asif, M., Nadeem, S., Jha, S., & Panjiyar, B. K. (2024). Neuropharmacology of Neuropathic Pain: A Systematic Review. Cureus, 16(9), e69028. https://doi.org/10.7759/cureus.69028
8. Mayo Clinic. (2024, July 20). How opioid addiction occurs. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/prescription-drug-abuse/in-depth/how-opioid-addiction-occurs/art-20360372
9 Czopek, A., Jończyk, J., Fryc, M., Kluzik, D., & Zagórska, A. (2025). Classic Psychedelics in Pain Modulation: Mechanisms, Clinical Evidence, and Future Perspectives. ACS Chemical Neuroscience, 16(12), 2163–2177. https://doi.org/10.1021/acschemneuro.5c00152
10 Zia, F. Z., Baumann, M. H., Belouin, S. J., Dworkin, R. H., Ghauri, M. H., Hendricks, P. S., Henningfield, J. E., Lanier, R. K., Ross, S., & Berger, A. (2023). Are psychedelic medicines the reset for chronic pain? Preliminary findings and research needs. Neuropharmacology, 233, 109528. https://doi.org/10.1016/j.neuropharm.2023.109528
11. Portenoy R. K. (2011). Treatment of cancer pain. Lancet (London, England), 377(9784), 2236–2247. https://doi.org/10.1016/S0140-6736(11)60236-5
12. Pathan, H., & Williams, J. (2012). Basic opioid pharmacology: an update. British journal of pain, 6(1), 11–16. https://doi.org/10.1177/2049463712438493
13. Goel, A., Rai, Y., Sivadas, S., Diep, C., Clarke, H., Shanthanna, H., & Ladha, K. S. (2023). Use of Psychedelics for Pain: A Scoping Review. Anesthesiology, 139(4), 523–536. https://doi.org/10.1097/ALN.0000000000004673
14. Ubhayarathna, Maleesha et al. “Molecular and structural insights into the 5-HT2C receptor as a therapeutic target for substance use disorders.” British Journal of Pharmacology, 181(22), 4414–4429. https://doi.org/10.1111/bph.16233
15. Treede, R.D., Rief, W., Barke, A., Aziz, Q., Bennett, M. I., Benoliel, R., Cohen, M., Evers, S., Finnerup, N. B., First, M. B., Giamberardino, M. A., Kaasa, S., Korwisi, B., Kosek, E., Lavand’homme, P., Nicholas, M., Perrot, S., Scholz, J., Schug, S., Smith, B. H., … Wang, S. J. (2019). Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain, 160(1), 19–27.https://doi.org/10.1097/j.pain.0000000000001384
16. Merskey H, Bogduk N. Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle: IASP Press; 1994.
17. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002.
18. Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87(1):3–11.
19. Miller RJ, Miller RE. Is osteoarthritis a disease of chronic inflammation? Clin Exp Rheumatol. 2017;35 Suppl 107(5):S1–S3.
20. Chang L, Naliboff BD, Mayer EA. Brain–gut interactions in irritable bowel syndrome. Gastroenterology. 2015;148(6):1355–1367.
21. Bates, D., Schultheis, B. C., Hanes, M. C., Jolly, S. M., Chakravarthy, K. V., Deer, T. R., Levy, R. M., & Hunter, C. W. (2019). A Comprehensive Algorithm for Management of Neuropathic Pain. Pain medicine (Malden, Mass.), 20(Suppl 1), S2–S12. https://doi.org/10.1093/pm/pnz075
22. Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120(11):3779–3787.
23. Baliki, M. N., & Apkarian, A. V. (2015). Nociception, Pain, Negative Moods, and Behavior Selection. Neuron, 87(3), 474–491. https://doi.org/10.1016/j.neuron.2015.06.005
24. Vadivelu, N., Kai, A. M., Kodumudi, V., Sramcik, J., & Kaye, A. D. (2018). The opioid crisis: A comprehensive overview. Current Pain and Headache Reports, 22(3), 16. https://doi.org/10.1007/s11916-018-0670-z
25. Pasternak, G. W., & Pan, Y. X. (2013). Mu opioids and their receptors: Evolution of a concept. Pharmacological Reviews, 65(4), 1257–1317. https://doi.org/10.1124/pr.112.007138
26. Volkow, N. D., & McLellan, A. T. (2016). Opioid abuse in chronic pain—Misconceptions and mitigation strategies. New England Journal of Medicine, 374(13), 1253–1263. https://doi.org/10.1056/NEJMra1507771
27. Kooijman, N. I. (2023). Are psychedelics the answer to chronic pain: A review. Frontiers in Pharmacology, 14, 711255. https://doi.org/10.3389/fphar.2021.711255
28. Preller, K. H., & Vollenweider, F. X. (2016). Phenomenology, structure, and dynamic of psychedelic states. Current Topics in Behavioral Neurosciences, 36, 221–256. https://doi.org/10.1007/7854 2016.459
29. Häuser, W., Sarac, A. J., & Üçeyler, N. (2021). Fibromyalgia as a central sensitization syndrome. Zeitschrift für Rheumatologie, 80(6), 522–527. https://doi.org/10.1007/s00393-021-01030-6
30. Schindler, E. A. D., Gottschalk, C. H., Weil, M. J., Shapiro, R. E., Wright, D. A., Sewell, R. A., & Ranganathan, M. (2015). Indoleamine hallucinogens in cluster headache: Results of the Clusterbusters Medication Use Survey. Journal of Psychoactive Drugs, 47(5), 376–381. https://doi.org/10.1080/02791072.2015.1107664
31. Johnson, M. W., Griffiths, R. R., Hendricks, P. S., & Henningfield, J. E. (2018). The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology, 142, 143–166. https://doi.org/10.1016/j.neuropharm.2018.05.012
32. Koseli, E., et al. (2025). IUPHAR Article: Psilocybin induces long-lasting effects via serotonin 2A receptor activation in chronic pain models. Neuropharmacology, 104, 1240-1249. https://doi.org/10.1016/j.neuropharm.2025.1041240
33. Robinson, C. L., Fonseca, A. C. G., Diejomaoh, E. M., D’Souza, R. S., Schatman, M. E., Orhurhu, V., & Emerick, T. (2024). Scoping Review: The Role of Psychedelics in the Management of Chronic Pain. Journal of pain research, 17, 965–973. https://doi.org/10.2147/JPR.S439348
34. Castellanos, J. P. (2020). Chronic pain and psychedelics: A review and proposedmechanism of action. Journal of Pain Research, 13, 123–135. https://doi.org/10.2147/JPR.S239847
35. Salinsky, L. M., Merritt, C. R., Zamora, J. C., Giacomini, J. L., Anastasio, N. C., & Cunningham, K. A. (2023). μ-opioid receptor agonists and psychedelics: pharmacological opportunities and challenges. Frontiers in pharmacology, 14, 1239159. https://doi.org/10.3389/fphar.2023.1239159
36. Schindler, E. A., et al. (2024). Repeat dosing of psilocybin in migraine headache: A randomized controlled trial. Journal of Clinical Psychopharmacology, 44(2), 123–130. https://doi.org/10.1097/JCP.0000000000001578
37. Kolbman, N., et al. (2023). Intravenous psilocybin attenuates mechanical hypersensitivity and thermal hyperalgesia in a rat model of chronic pain. Current Biology, 33(5), 123–130. https://doi.org/10.1016/j.cub.2022.12.024
38. Glynos, N. G., et al. (2025). Psychedelics and chronic pain: Self-reported outcomes on the use of psychedelics for chronic pain management. Journal of Pain Research, 18, 11811946. https://doi.org/10.2147/JPR.S11811946
39. Aday, J. S., et al. (2025). Preliminary safety and effectiveness of psilocybin-assisted therapy for chronic pain. Frontiers in Pain Research, 6, 1527783. https://doi.org/10.3389/fpain.2025.1527783
40. Cavarra, M., et al. (2025). A randomized placebo-controlled study of the effects of LSD on pain perception. Heliyon, 11(5), e10432. https://doi.org/10.1016/j.heliyon.2024.e10432
41. Schlag, A. K., Aday, J., Salam, I., Neill, J. C., & Nutt, D. J. (2022). Adverse effects of psychedelics: From anecdotes and misinformation to systematic science. Journal of psychopharmacology (Oxford, England), 36(3), 258–272. https://doi.org/10.1177/02698811211069100
42. Simonsson O, Johnson MW, Hendricks PS. Psychedelic and MDMA-Related Adverse Effects—A Call for Action. JAMA Health Forum. 2024;5(11):e243630. doi:10.1001/jamahealthforum.2024.3630
About the author

Emerson G. Brown
Emerson Brown is a student researcher with strong interests in psychology, chemistry, and neurobiology. She has conducted independent research under the mentorship of Dr. Adam Behensky, focusing on the intersection of neuroscience and medicine. Beyond her academic studies, she is an active competitor in Science Olympiad, where she explores scientific inquiry in a team-based setting. Emerson is passionate about medicine and aspires to contribute to advancements in patient care and biomedical research.