
Author: Madeline Wiley
Mentor: Dr. Ana Maria Ortega-Prieto
Westtown School
Abstract
Interferons (IFNs) are a group of proteins produced by cells in response to viral infections, making them a crucial aspect of the immune system. They essentially regulate the immune system and how it responds to threats by signaling when a pathogen is present, interfering with viral replication and triggering various defense mechanisms. While IFNs are often protective, their effects are not universally beneficial. In certain contexts, particularly chronic infections, prolonged IFN signaling can suppress immunity, cause tissue damage, and contribute to disease severity. This paper explores the dual role of IFN responses in both acute and chronic infections, specifically focusing on influenza A, HCV , HIV , and SARS-CoV-2. By examining both the protective and pathological impacts of IFN activity, this review highlights IFN signaling as a double edged sword and underscores the need for more targeted strategies in the future.
Between Shield and Sword: How Interferons Protect and Harm the Host Immune System
Interferons are vitally important for defending the body against viruses, yet in some infections, they can become problematic. IFNs are a type of cytokine, which are signaling proteins that aid communication between cells during immune responses (Isaacs et al., 1957). IFNs are critical in the innate immune system, which is the first line of non-specific defense against pathogens. The IFN pathway is indispensable for the innate immune system response because IFNs antagonize pathogens, which limits infection and its spread. IFNs essentially are messengers in the immune system, as they alert cells to the presence of a virus and allow them to trigger antiviral defenses (Katze et al., 2002). They are particularly helpful because many IFNs can be found at mucosal surfaces, where disease prevention is key. They help detect viral threats and facilitate the activation and production of antiviral defenses and immune pathways, therefore proving themselves critical in the recovery from viral infections.
Most cells in the body may produce IFNs in response to a viral infection, including lymphocytes (Fenner et al., 1987). Lymphocytes produce many IFNs and are the main source of their circulation when a virus is present and replicating in the bloodstream. IFNs are formed when they bind viral molecules to cell surface or intracellular pattern recognition receptors and they are released by the host cell in response to the presence of viruses. This binding signals the transcription and secretion of IFNs (Katze et al., 2002). They can be made in a laboratory to be used as treatment as well (Gibbert et al., 2012). There are three main types of IFNs—type I, II, and III—that aid the immune system in different functions.
Type I IFNs include IFN-α, IFN-β, IFN-ε, IFN-κ, IFN-ω, IFN-δ, IFN-ζ, and IFN-τ and they bind to specific surface cell receptors, IFN-α/β (Murira et al., 2016). They are produced after pattern-recognition receptor (PRR) stimulation as part of the innate immune response 4 (Walker et al., 2021). The IFN wave produced by this receptor induces IRF7 phosphorylation and causes a positive feedback loop. This wave releases type I IFNs that signal through the IFN-α/β receptors which then signal in the janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway (Lee et al., 2018). These IFNs inactivate eukaryotic translation initiation factor 2a (elF-2α), which inhibits viral protein synthesis. They also activate RNase L using the interferon stimulated gene (ISG) oligoadenylate synthetase (OAS), which splits any ssRNA in the cytoplasm of the infected cell, also inhibiting viral replication (Cusic et al., 2024). They are released early on in viral infection and provide direct antiviral effects and innate immune activation. They induce more than 300 ISGs which block viral replication and stimulate NK cells (Lee et al., 2018).
Type II IFNs are much different than other IFNs, which is why types I and III are at the center of this review. Type II IFNs include IFN-γ and they signal through a different receptor than other IFN types, known as the IFN-γ receptor complex, which is why only some cells respond to these IFNs (Lee et al., 2018). They are mainly produced by NK cells or innate lymphoid type 1 cells, but not directly by virus infected cells, which is another reason why they are not further discussed in this review (Kim et al., 2021). Their main function is to regulate innate immunity and activate other immune cells, such as macrophages. They enhance the body’s adaptive immune response, whereas other IFNs elicit a generalized response to pathogens (Platanias, 2005).
Type III IFNs include IFN-λ1, IFN-λ2, IFN-λ3, and IFN-λ4 and they are similar to type I IFNs in the way they inhibit viral infections. They are produced mainly by plasmacytoid dendritic cells. They bind to the receptors IFRL1 and IL-10R2 and they are synthesized when the host cell detects pathogen-associated molecular patterns and also signals through the JAK/STAT 5 pathway, similar to type I IFNs (Chyuan et al., 2019). Unlike type I IFNs, type III IFNs mainly help maintain healthy mucosal surfaces and control viral infections there. They also target the human liver, induce antiviral state in hepatocytes, and can impair the clearance of HCV . Only some cells respond to type III IFNs, unlike how most respond to type I (Odendall et al., 2015).
As mentioned earlier, JAK/STAT pathway is an IFN signaling pathway shared by both type I and III IFNs. 50+ cytokines and growth factors are found in the JAK/STAT pathway, including IFNs. JAK/STAT mediates events including apoptosis, inflammation, tissue repair, hematopoiesis, immune fitness, and adipogenesis (Hu et al., 2021). Upon ligand binding, JAK trans-phosphorylation is triggered by receptor dimerization. This leads to phosphorylation of receptor tyrosines and then to recruitment of STAT proteins (STAT1–6). Phosphorylated STATs then form homo/heterodimers and they then enter the nucleus and regulate target gene transcription.
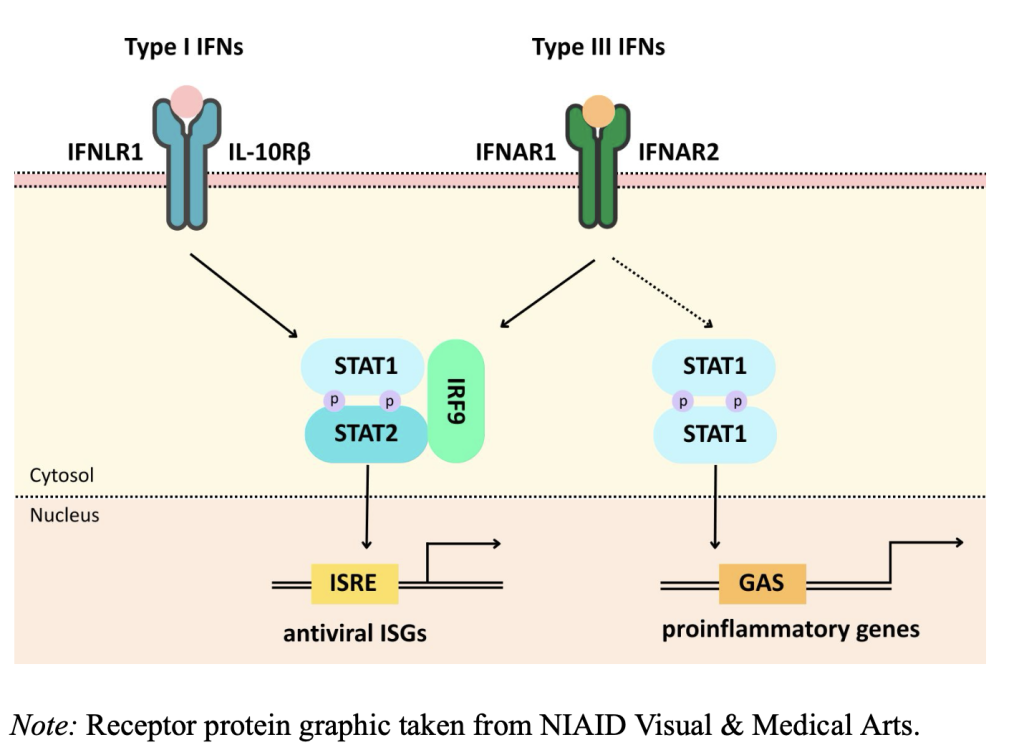
Figure 1 The signaling pathways of type I and III IFNs
Innate Immune Sensing
The innate immune system is characterized by its production of inflammatory cytokines that lead to inflammatory cell activation/recruitment. Innate immune cells have pattern recognition receptors (PRRs) which sense pathogens (Uehata et al., 2020). The best characterized PRRs are toll-like receptors (TLRs) which recognize many pathogens. As seen in figure 2, different types of TLRs have different specialties. TLR 3 recognizes double stranded (ds) RNA and viral replication intermediates localized in endosomes. TLR 7/8 recognizes single stranded (ss) RNA and viral genomes localized in endosomes. TLR 9 recognizes CpG DNA and DNA viruses localized in endosomes. TLR 2/4 recognizes viral glycoproteins and some enveloped viruses localized at the cell surface (Kawai et al., 2010). Essentially, Viral nucleic acids are taken up into endosomes, where TLRs detect them.
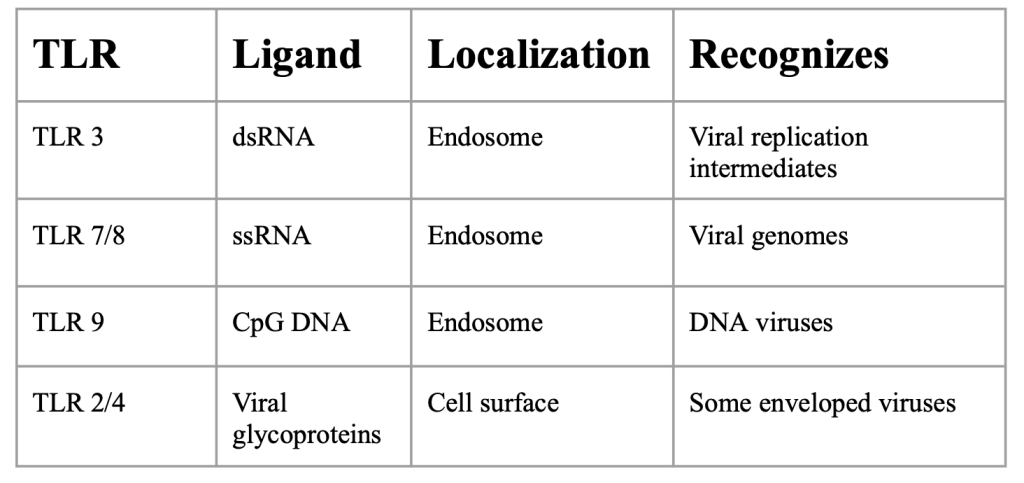
Figure 2: TLR overview
Retinoic acid-inducible gene I (RIG-I) receptors are another type of PRR that contribute to inflammatory responses by activating downstream signaling pathways. RIG-I recognizes short dsDNA with 5’ triphosphate ends and ssRNA from negative sense RNA viruses (Yoneyama et al., 2004). TLRs and RIG-I like receptors (RLRs) activate signal pathways and downstream signaling modules that then activate NF-κB, AP-1, and IRF3/7 that induce inflammatory cytokines (Uehata et al., 2020). ISGs also play a role in innate immune sensing. PRRs, JAKs, and STATs are ISGs. ISGs control infections by directly targeting functions and pathways that are required during pathogen life cycles (Schneider et al., 2015). They enhance the detection of pathogens using PRRs and refine the signaling machinery of IFN pathways, allowing for effective and regulated immune responses.
While IFNs are generally protective, their impact can vary dramatically based on timing, context, and infection type. In some situations, particularly in chronic or severe infections, IFN responses can become dysregulated, leading to excess inflammation and immune suppression, along with other damages. This dual role of interferons, as both defenders and potential damagers, forms the focus of this paper.
Type I IFNs in Acute Infections
Influenza A
Type I IFNs are known to be critical in acute infections, such as influenza or SARS-CoV-2, but they also are attributed to various negative side effects. Specifically in influenza A, early type I IFN response demonstrates how type I IFNs can have protective antiviral effects but can also contribute to immunopathology (Wu et al., 2020). Immunopathology is adverse effects generated by the immune response (Marshall et al., 2018). It is known that type I IFNs are only effective against influenza in mice if Myxovirus-resistance protein (Mx1), an IFN-induced resistance factor, is present (Abebaw et al., 2024). In a 2007 study conducted by Koerner et al., they generated mutated mice, two of the types being Mx-wild type (wt) and Mx-BKO. Mx-wt mice had both functional IFN-β and Mx1 capabilities, while Mx-BKO mice had functional Mx1 but no IFN-β. Both types of mice were infected with influenza A via injection, as shown in figure 3. Through their studies, they found that IFN-β has a relevant role in early antiviral defense. Mx-BKO mice were significantly more susceptible to influenza A infection compared to Mx-wt mice. These mice allowed lots more viral growth in lab tests, especially when infected through the nose. This was because the 50% lethal dose was 20x lower in Mx-BKO mice compared to Mx-wt mice. In comparison, the Mx-wt mice were highly resistant to the virus and survived high doses of the virus that killed Mx-BKO mice. Their immune system was able to stop the virus from replicating efficiently because they produced IFN-I that induces ISGs that suppress replication in lung epithelial cells. This study illustrated how IFN-β is key in suppressing acute viral replication and without it, the body’s defenses are compromised.
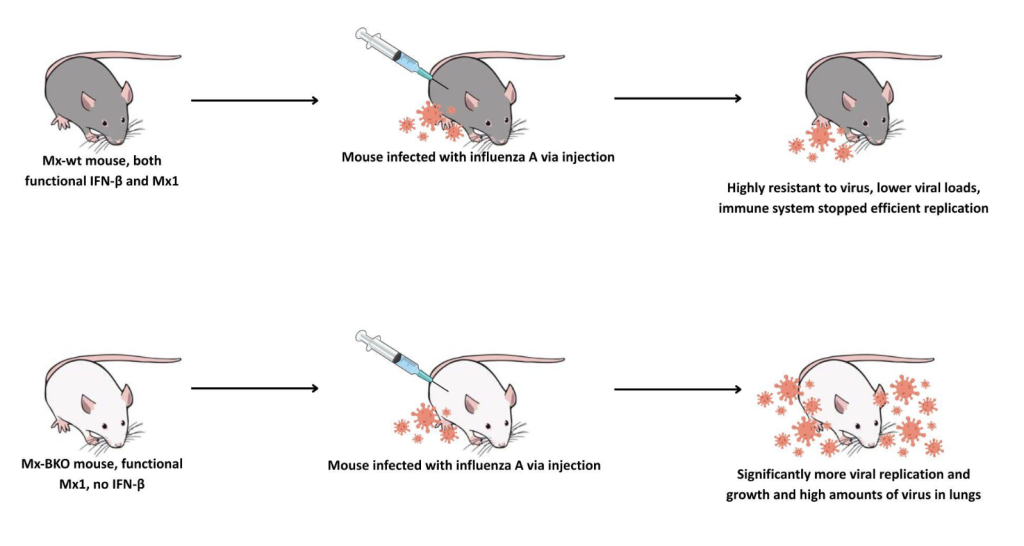
Figure 3: IFN-I in Influenza A Note: Based on a 2007 study by Koerner et al. Syringe and lab mouse graphic taken from NIAID Visual & Medical Arts.
While this study showed the beneficial effects of type I IFNs in influenza A infection, they also hold a negative role. In a 2012 study conducted by Li et al., it was discovered that IFN-I induction during influenza A infection increases susceptibility to Secondary Streptococcus pneumoniae. The majority of deaths after influenza A infection in influenza pandemics are caused by secondary bacterial pneumonia superinfection, illustrating the importance of the role IFN-I plays (McCullers, 2006). In this study, wild type (WT) mice and mice lacking IFNAR (IFNAR KO) were infected with S. pneumoniae following influenza A infection. The WT faced 10 a deadly secondary Streptococcus pneumoniae infection and IFNAR KO mice successfully cleared the infection, proving how IFN-I induction leads to susceptibility.
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
SARS-CoV-2 is the virus that causes coronavirus disease 2019 (COVID-19). Late type I IFN response in SARS-CoV-2 also highlights how improper timing can turn IFNs into contributors of immunopathology. While mutations in the type I IFN pathway have been linked to life threatening symptoms in SARS-CoV-2 infection, they have also been linked to cytokine storms (Kim et al., 2021). A cytokine storm is a hyperinflammatory reaction where the body releases too many cytokines into the blood too quickly (Zanza et al., 2022). It manifests similarly to influenza and can lead to organ failure, coagulopathy and death (Fajgenbaum et al., 2020). Cytokine storms have been determined to be a key pathogenic factor in SARS-CoV-2 infection (King et al., 2021). Various studies have discovered the increased presence of inflammatory cytokines such as IL-1β, IL-2, IL-6, IL-10, and IFN-γ in SARS-CoV-2 patients (Zanza et al., 2022). These increased levels of cytokines are associated with disease severity (Brunet-Ratnasingham et al., 2024). Severely ill COVID-19 patients showed an impaired early IFN response which was followed by a sustained type I IFN storm. This was linked to hyperinflammation and acute respiratory distress syndrome (ARDS) (Hadjadj et al., 2020).
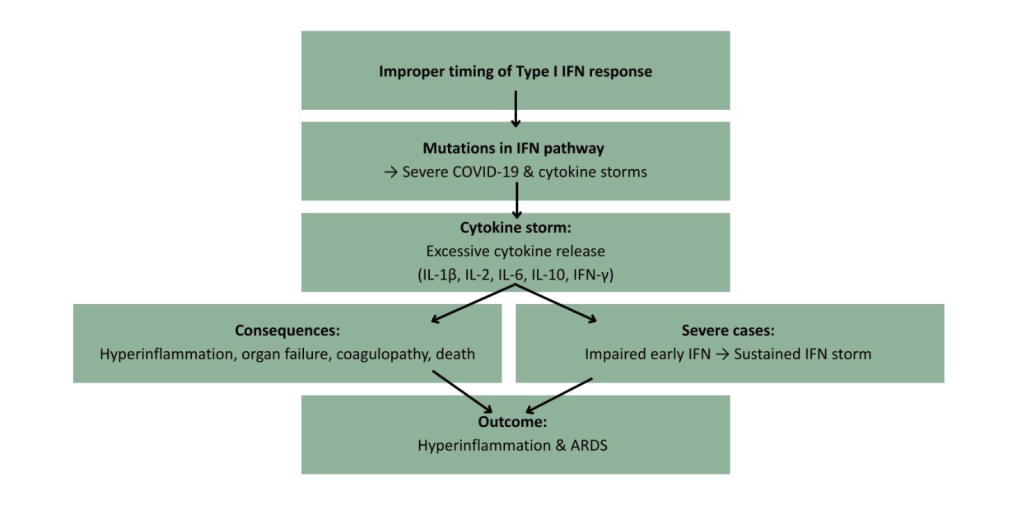
Figure 4 Type I IFN Dysregulation and Cytokine Storm in SARS-CoV-2 11
Type I IFNs in Chronic Infections
The presence of type I IFNs in chronic infections, such as HCV or HIV , is notable as well.
Human Immunodeficiency Virus (HIV)
The dual role of IFN-I is also highlighted during HIV infection. Although HIV activates host immune defenses, IFN-I and ISGs are ultimately insufficient to eradicate the virus. In the acute phase of infection, IFN-I hinders HIV-1 spread by stimulating key immune defenses, including ISGs that help contain the virus (Cheng et al., 2017). Despite this though, during chronic HIV infection, prolonged type I IFN exposure has been linked detrimental effects such as desensitization, detrimental hyperimmune activation leading to disease progression, impaired viral control, and upregulation of exhaustion markers on T cells (Scagnolari et al., 2018 and Le Saout et al., 2014). HIV also evades IFN-I’s defense using multiple tactics such as downregulating key signaling factors IRF3, RIG-I, PKR, and MHC-I via viral proteins like Vpr, protease, Tat, and Nef, therefore disrupting IFN-I pathways (Ranganath et al., 2016). In addition, 12 IFN-I can inadvertently induce apoptosis of uninfected CD4⁺ T cells via TRAIL-DR5 signaling, further accelerating immune depletion (Herbeuval et al., 2007). From a therapeutic standpoint, some experimental models suggest that blocking IFN-I signaling during chronic HIV infection in combination with antiretroviral therapy (ART) can diminish immune activation, revitalize CD8⁺ T cell responses, reduce viral reservoirs, and improve viral suppression (Zhen et al., 2016). This highlights the potential benefits of modulating IFN-I dynamics in treated HIV patients. Overall, the role of IFN-I in HIV infection highlights chronic exposure and how timing and duration of IFN responses shape outcome.
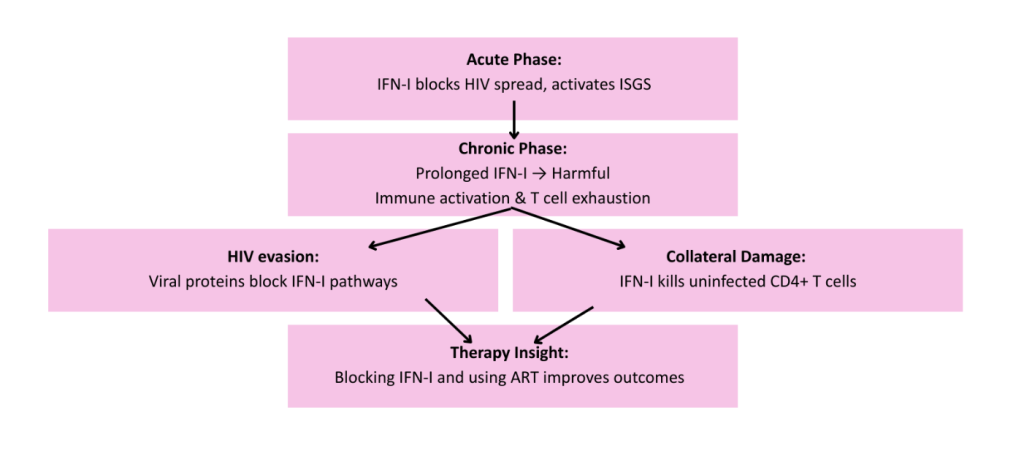
Figure 5: Type I IFNs in HIV Infection
Hepatitis C
Virus (HCV) Pegylated interferon and ribavirin treatment has become standard for treating HCV , mainly due to its pharmacokinetic properties which allow for less frequent dosing compared to conventional IFN-α (Aghemo et al., 2010). This treatment is made by chemically attaching polyethylene glycol (PEG) chains to interferon molecules (Friedman et al., 2010). Pegylated IFN-α treatment exerts direct antiviral effects by inducing ISGs that block viral replication and 13 also enhances adaptive and innate immunity, including the upregulation of major histocompatibility complex (MHC) class I and II molecules, encoding cell surface proteins crucial for the immune system, antigen presentation and increased T cell activation (Dill et al., 2014). Combining IFN-α with ribavirin, an antiviral nucleoside analog, has been found to enhance treatment effectiveness (Friedman et al., 2010). This treatment supports the idea that IFNs can be successful protectors against viruses.
Type III IFNs in Infections
IFN λ-3 and λ-4 in HCV are great examples of the effects of type III IFNs in infections. In HCV infection, IFN-λ3 is linked with clearance of HCV infection as well as a protective antiviral role in the liver (Liu et al., 2015). It is inducible during viral infection and enhances innate antiviral defense without triggering widespread inflammation. IFN λ-4 is linked with impaired clearance of HCV infection (Boisvert et al., 2016). It is known to attenuate HCV-specific T cell responses that leads to a negative impact on cellular immunity (Chen et al., 2021). Production of IFN-λ4 triggers chronic inflammation and persistent ISG expression which can inhibit an effective immune response. It can also lead to a pre-exhausted immune state which can limit the effectiveness of other IFN therapies (Prokunina-Olsson et al., 2013). IFN-λ4 is also only found in ΔG carriers (O’Brien et al., 2014). Some people clear HCV faster than others and this is mostly due to genetic variation. The IFN-λ3 and IFN-λ4 location on chromosome 19 shows single nucleotide polymorphisms (SMPs) linked to HCV outcomes (Ge et al., 2009). One of these SNPs is at Rs12979860 in IFN-λ3. This SNP is highly linked to the spontaneous clearance of HCV without treatment and improved response to therapy. There are three different genotypes individuals may possess that are relevant to this SNP: CC, TT, or CT. Individuals with the CC genotype have higher IFN-λ3 expression which leads to stronger ISG activation and 14 better viral clearance. Those with the CT or TT genotype are less likely to clear the virus naturally or respond well to older treatments (Wu et al., 2015). Rs368234815 in IFN-λ4 is another SNP linked to HCV outcomes. Those with the TT genotype do not produce IFN-λ4 and typically clear the virus more efficiently. Those with the other genotype, ΔG, produce IFN-λ4 which makes it harder to clear the virus. These SNPs illustrate how some IFNs, such as IFN-λ4, can be inherently maladaptive.
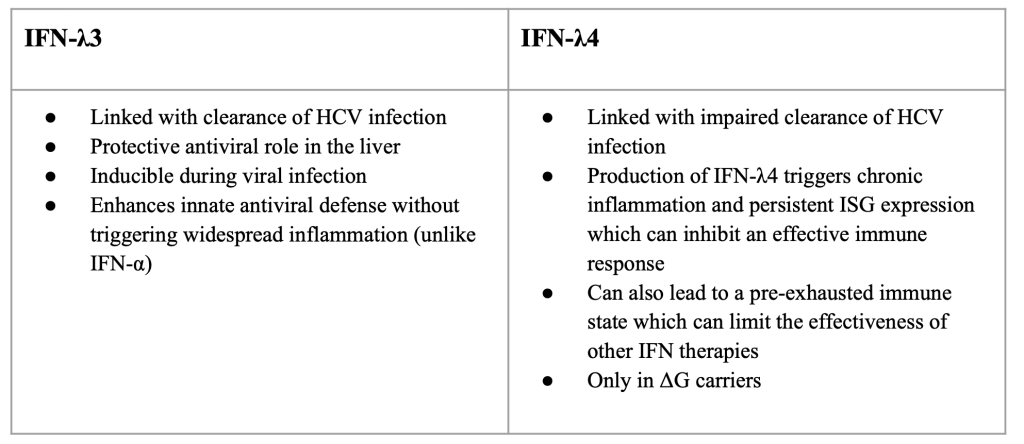
Figure 6: IFN-λ3 and λ4 in HCV infection
Conclusion
Interferon signaling is regarded as one of the immune system’s most powerful antiviral tools, yet its strength makes it a double edged sword. On one side, rapid IFN responses promote viral clearance, activate innate and adaptive immunity, and protect mucosal surfaces. On the other, sustained or dysregulated IFN signaling can promote chronic inflammation, tissue damage, and 15 immune exhaustion, as seen in infections like HCV or SARS-CoV-2. Recognizing the duality of IFN signaling underscores the importance of context, timing, and regulation in immune responses and defense. As research advances, the challenge will be to maintain IFN’s protective qualities in therapies while minimizing their pathological consequences.
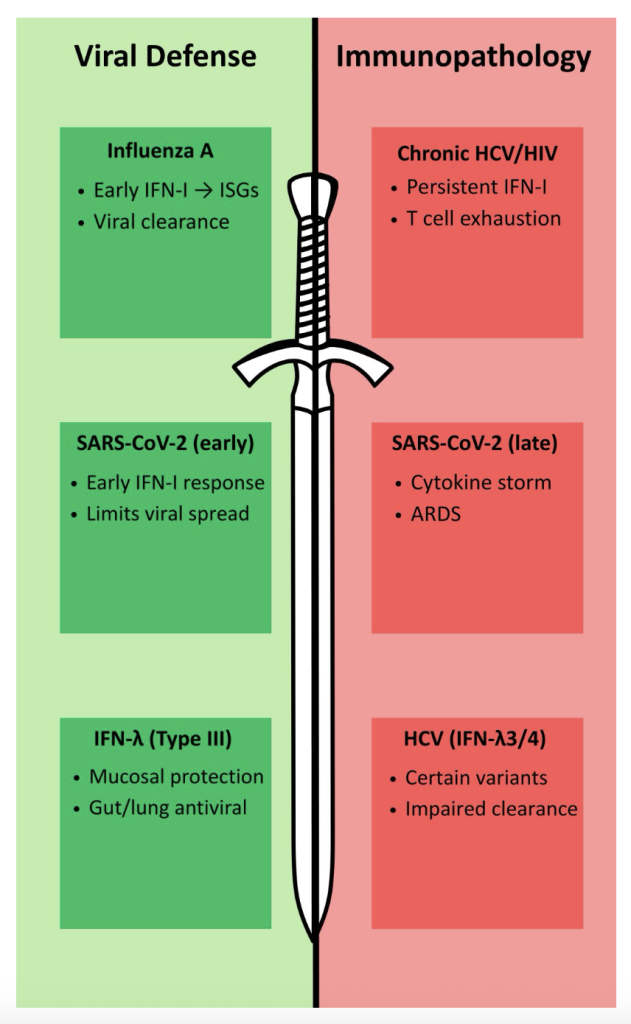
Figure 7: Summary of the role of IFNs in viral infections
References
Abebaw, D., Akelew, Y ., Adugna, A., Teffera, Z. H., Belew, H., Selabat, B., Getie, M., Mulu, A. T., & Atnaf, A. (2024). Recent updates of interferon-derived myxovirus resistance protein A as a biomarker for acute viral infection. European journal of medical research, 29(1), 612. https://doi.org/10.1186/s40001-024-02221-8
Aghemo, A., Rumi, M. G., & Colombo, M. (2010). Pegylated interferons α2a and α2b in the treatment of chronic hepatitis C. Nature Reviews Gastroenterology & Hepatology, 7(9), 485–494. https://doi.org/10.1038/nrgastro.2010.101
Boisvert, M., & Shoukry, N. H. (2016). Type III interferons in hepatitis C virus infection. Frontiers in Immunology, 7. https://doi.org/10.3389/fimmu.2016.00628
Brunet-Ratnasingham, E., Morin, S., Randolph, H. E., Labrecque, M., Bélair, J., Lima-Barbosa, R., Pagliuzza, A., Marchitto, L., Hultström, M., Niessl, J., Cloutier, R., Sreng Flores, A. M., Brassard, N., Benlarbi, M., Prévost, J., Ding, S., Anand, S. P., Sannier, G., Marks, A., . . . Vezina, D. (2024). Sustained IFN signaling is associated with delayed development of sars-cov-2-specific immunity. Nature Communications, 15(1). https://doi.org/10.1038/s41467-024-48556-y
Chen, Q., Coto-Llerena, M., Suslov, A., Teixeira, R. D., Fofana, I., Nuciforo, S., Hofmann, M., Thimme, R., Hensel, N., Lohmann, V ., Ng, C. K. Y ., Rosenberger, G., Wieland, S., & Heim, M. H. (2021). Interferon lambda 4 impairs hepatitis C viral antigen presentation and attenuates T cell responses. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-25218-x
Cheng, L., Yu, H., Li, G., Li, F., Ma, J., Li, J., Chi, L., Zhang, L., & Su, L. (2017). Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction 17 during chronic hiv-1 infection. JCI Insight, 2(12). https://doi.org/10.1172/jci.insight.94366
Chyuan, I.-T., Tzeng, H.-T., & Chen, J.-Y . (2019). Signaling pathways of type I and type III interferons and targeted therapies in systemic lupus erythematosus. Cells, 8(9), 963. https://doi.org/10.3390/cells8090963
Cusic, R., & Burke, J. M. (2024). Condensation of rnase L promotes its rapid activation in response to viral infection in mammalian cells. Science Signaling, 17(837). https://doi.org/10.1126/scisignal.adi9844
Dill, M. T., Makowska, Z., Trincucci, G., Gruber, A. J., V ogt, J. E., Filipowicz, M., Calabrese, D., Krol, I., Lau, D. T., Terracciano, L., van Nimwegen, E., Roth, V ., & Heim, M. H. (2014). Pegylated ifn-α regulates hepatic gene expression through transient jak/stat activation. Journal of Clinical Investigation, 124(4), 1568-1581. https://doi.org/10.1172/jci70408
Fajgenbaum, D. C., & June, C. H. (2020). Cytokine storm. New England Journal of Medicine, 383(23), 2255-2273. https://doi.org/10.1056/nejmra2026131
Fenner, F., Bachmann, P. A., GIbbs, E. P. J., Murphy, F. A., Studdert, M. J., & White, D. O. (1987). Determinants of host resistance. In Veterinary virology (pp. 153-159). Academic Press. https://doi.org/10.1016/B978-0-12-253055-5.50012-8
Friedman, R. M., & Contente, S. (2010). Treatment of hepatitis C infections with interferon: A historical perspective. Hepatitis Research and Treatment, 2010, 1-4. https://doi.org/10.1155/2010/323926
Ge, D., Fellay, J., Thompson, A. J., Simon, J. S., Shianna, K. V ., Urban, T. J., Heinzen, E. L., Qiu, P., Bertelsen, A. H., Muir, A. J., Sulkowski, M., McHutchison, J. G., & Goldstein, 18 D. B. (2009). Genetic variation in il28b predicts hepatitis C treatment-induced viral clearance. Nature, 461(7262), 399-401. https://doi.org/10.1038/nature08309
Gibbert, K., Schlaak, J., Yang, D., & Dittmer, U. (2013). IFN-α subtypes: Distinct biological activities in anti-viral therapy. British Journal of Pharmacology, 168(5), 1048-1058. https://doi.org/10.1111/bph.12010
Hadjadj, J., Yatim, N., Barnabei, L., Corneau, A., Boussier, J., Smith, N., Péré, H., Charbit, B., Bondet, V ., Chenevier-Gobeaux, C., Breillat, P., Carlier, N., Gauzit, R., Morbieu, C., Pène, F., Marin, N., Roche, N., Szwebel, T.-A., Merkling, S. H., . . . Kernéis, S. (2020). Impaired type I interferon activity and inflammatory responses in severe covid-19 patients. Science, 369(6504), 718-724. https://doi.org/10.1126/science.abc6027
Herbeuval, J.-P., & Shearer, G. M. (2007). HIV-1 immunopathogenesis: How good interferon turns bad. Clinical Immunology, 123(2). https://doi.org/10.1016/j.clim.2006.09.016
Hu, X., Li, J., Fu, M., Zhao, X., & Wang, W. (2021). The JAK/STAT signaling pathway: From bench to clinic. Signal Transduction and Targeted Therapy, 6(1). https://doi.org/10.1038/s41392-021-00791-1
Isaacs, A., & Lindenmann, J. (1957). Virus interference. I. the interferon. Proceedings of the Royal Society of London. Series B – Biological Sciences, 147(927), 258-267. https://doi.org/10.1098/rspb.1957.0048
Katze, M. G., He, Y ., & Gale, M. (2002). Viruses and interferon: A fight for supremacy. Nature Reviews Immunology, 2(9), 675-687. https://doi.org/10.1038/nri888
Kawai, T., & Akira, S. (2010). The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nature Immunology, 11(5), 373-384. https://doi.org/10.1038/ni.1863 19
Kim, Y .-M., & Shin, E.-C. (2021). Type I and III interferon responses in sars-cov-2 infection. Experimental & Molecular Medicine, 53(5), 750-760. https://doi.org/10.1038/s12276-021-00592-0
King, C., & Sprent, J. (2021). Dual nature of type I interferons in sars-cov-2-induced inflammation. Trends in Immunology, 42(4), 312-322. https://doi.org/10.1016/j.it.2021.02.003
Koerner, I., Kochs, G., Kalinke, U., Weiss, S., & Staeheli, P. (2007). Protective role of beta interferon in host defense against influenza A virus. Journal of Virology, 81(4), 2025-2030. https://doi.org/10.1128/jvi.01718-06
Lee, A. J., & Ashkar, A. A. (2018). The dual nature of type I and type II interferons. Frontiers in Immunology, 9. https://doi.org/10.3389/fimmu.2018.02061
Le Saout, C., Hasley, R. B., Imamichi, H., Tcheung, L., Hu, Z., Luckey, M. A., Park, J.-H., Durum, S. K., Smith, M., Rupert, A. W., Sneller, M. C., Lane, H. C., & Catalfamo, M. (2014). Chronic exposure to type-i IFN under lymphopenic conditions alters cd4 T cell homeostasis. PLoS Pathogens, 10(3), e1003976. https://doi.org/10.1371/journal.ppat.1003976
Li, W., Moltedo, B., & Moran, T. M. (2012). Type I interferon induction during influenza virus infection increases susceptibility to secondary streptococcus pneumoniae infection by negative regulation of γδ T cells. Journal of Virology, 86(22), 12304-12312. https://doi.org/10.1128/jvi.01269-12
Liu, B., McGilvray, I., & Chen, L. (2015). IFN-λ: A new class of interferon with distinct functions-implications for hepatitis C virus research. Gastroenterology Research and Practice, 2015, 1-9. https://doi.org/10.1155/2015/796461
Marshall, J. S., Warrington, R., Watson, W., & Kim, H. L. (2018). An introduction to immunology and immunopathology. Allergy, Asthma & Clinical Immunology, 14(S2). https://doi.org/10.1186/s13223-018-0278-1
McCullers, J. A. (2006). Insights into the interaction between influenza virus and pneumococcus. ASM Journals. https://doi.org/10.1128/cmr.00058-05
Murira, A., & Lamarre, A. (2016). Type-I interferon responses: From friend to foe in the battle against chronic viral infection. Frontiers in Immunology, 7. https://doi.org/10.3389/fimmu.2016.00609
NIAID Visual & Medical Arts. (10/7/2024). Syringe. NIAID NIH BIOART Source. bioart.niaid.nih.gov/bioart/505
NIAID Visual & Medical Arts. (10/7/2024). Lab Mouse. NIAID NIH BIOART Source. bioart.niaid.nih.gov/bioart/281
NIAID Visual & Medical Arts. (10/7/2024). Receptor Protein. NIAID NIH BIOART Source. bioart.niaid.nih.gov/bioart/439
O’Brien, T. R., Prokunina-Olsson, L., & Donnelly, R. P. (2014). IFN-λ4: The paradoxical new member of the interferon lambda family. Journal of Interferon & Cytokine Research, 34(11), 829-838. https://doi.org/10.1089/jir.2013.0136
Odendall, C., & Kagan, J. C. (2015). The unique regulation and functions of type III interferons in antiviral immunity. Current Opinion in Virology, 12, 47-52. https://doi.org/10.1016/j.coviro.2015.02.003
Platanias, L. C. (2005). Mechanisms of type-I- and type-II-interferon-mediated signalling. Nature Reviews Immunology, 5(5), 375-386. https://doi.org/10.1038/nri1604 21
Prokunina-Olsson, L., Muchmore, B., Tang, W., Pfeiffer, R. M., Park, H., Dickensheets, H., Hergott, D., Porter-Gill, P., Mumy, A., Kohaar, I., Chen, S., Brand, N., Tarway, M., Liu, L., Sheikh, F., Astemborski, J., Bonkovsky, H. L., Edlin, B. R., Howell, C. D., . . . O’Brien, T. R. (2013). A variant upstream of ifnl3 (IL28B) creating a new interferon gene ifnl4 is associated with impaired clearance of hepatitis C virus. Nature Genetics, 45(2), 164-171. https://doi.org/10.1038/ng.2521
Ranganath, N., Sandstrom, T. S., Fadel, S., Côté, S. C., & Angel, J. B. (2016). Type I interferon responses are impaired in latently HIV infected cells. Retrovirology. https://doi.org/10.1186/s12977-016-0302-9
Scagnolari, C., & Antonelli, G. (2018). Type I interferon and hiv: Subtle balance between antiviral activity, immunopathogenesis and the microbiome. Cytokine & Growth Factor Reviews, 40, 19-31. https://doi.org/10.1016/j.cytogfr.2018.03.003
Schneider, W. M., Chevillotte, M. D., & Rice, C. M. (2014). Interferon-Stimulated genes: A complex web of host defenses. Annual Review of Immunology, 32(1), 513-545. https://doi.org/10.1146/annurev-immunol-032713-120231
Uehata, T., & Takeuchi, O. (2020). RNA recognition and immunity—innate immune sensing and its posttranscriptional regulation mechanisms. Cells, 9(7), 1701. https://doi.org/10.3390/cells9071701
Walker, F. C., Sridhar, P. R., & Baldridge, M. T. (2021). Differential roles of interferons in innate responses to mucosal viral infections. Trends in Immunology, 42(11), 1009-1023. https://doi.org/10.1016/j.it.2021.09.003
Wu, Q., Wang, C., Chen, E. Q., Tang, H., Li, Z. Z., & Lei, X. Z. (2015). Interferon lambda 4 polymorphism predicts sustained viral response in hepatitis C virus patients irrespective 22 of hepatitis C virus genotypes, ethnicity or treatment regimen: Results from a meta-analysis. Hepatitis Monthly, 15(12). https://doi.org/10.5812/hepatmon.32707
Wu, W., & Metcalf, J. P. (2020). The role of type I ifns in influenza: Antiviral superheroes or immunopathogenic villains? Journal of Innate Immunity, 12(6), 437-447. https://doi.org/10.1159/000508379
Yoneyama, M., Kikuchi, M., Natsukawa, T., Shinobu, N., Imaizumi, T., Miyagishi, M., Taira, K., Akira, S., & Fujita, T. (2004). The RNA helicase rig-i has an essential function in double-stranded rna-induced innate antiviral responses. Nature Immunology, 5(7), 730-737. https://doi.org/10.1038/ni1087
Zanza, C., Romenskaya, T., Manetti, A. C., Franceschi, F., La Russa, R., Bertozzi, G., Maiese, A., Savioli, G., V olonnino, G., & Longhitano, Y . (2022). Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina (Kaunas, Lithuania), 58(2), 144. https://doi.org/10.3390/medicina58020144
Zhen, A., Rezek, V ., Youn, C., Lam, B., Chang, N., Rick, J., Carrillo, M., Martin, H., Kasparian, S., Syed, P., Rice, N., Brooks, D. G., & Kitchen, S. G. (2016). Targeting type I interferon–mediated activation restores immune function in chronic HIV infection. Journal of Clinical Investigation, 127(1), 260-268. https://doi.org/10.1172/jci89488
About the author

Madeline Wiley
Madeline is a senior at Westtown School in Pennsylvania with a strong interest in biology and chemistry, particularly their intersection in the field of pharmacology. Her fascination with how these disciplines explain disease and drive medical innovation led her to explore topics such as molecular biology, pharmacokinetics, and the pharmaceutical industry. Over time, this curiosity grew into a deep desire to contribute original research and become part of the discovery process for new therapies.
Madeline is passionate about addressing real-world challenges in public health, illness, and medicine. Outside of her scientific interests, she enjoys playing guitar and piano, singing, and performing at local venues.