Author: Sofia Sattler
Mentor: Dr. Hong Pan
Franklin Delano Roosevelt
Highlights
- Early stages of Alzheimer’s are critical for identifying and stopping the disease’s progression, offering a window for potential intervention.
- Environmental factors such as pollutants and chemical waste have been found to significantly contribute to the risk of developing Alzheimer’s
- Advanced medical imaging techniques play a pivotal role in detecting Alzheimer’s biomarkers facilitating early diagnosis.
- AI’s emergence in diagnostic processes and the push towards personalized medicine may represent the frontier in combating Azlheimer’s promising more targeted and effective therapies.
Abstract
In navigating the complexities of Alzheimer’s Disease (AD), this review emphasizes the critical role of early detection in altering the course of this prevalent neurodegenerative disorder. With dementia affecting approximately 50 million individuals globally and AD being the predominant form, the urgency of diagnosing AD in its early stages—Subjective Cognitive Decline (SCD) and Mild Cognitive Impairment (MCI)—cannot be overstated. Identifying AD early opens the door to diagnostic and therapeutic strategies that can substantially slow or even halt the progression of the disease.
This review begins with a introduction to Alzheimer’s Disease, giving a picture of its clinical and pathological landscape. The discussion then moves to the crucial early stages of AD, Subjective Cognitive Decline and Mild Cognitive Impairment, highlighting how these early warning signs connect to the more severe stages of the disease. The review offers a dive into the causes of AD, particularly focusing on the build-up of chemical waste in the brain. It also explores the genomic landscape of AD with a special focus on the Apolipoprotein E (APOE) gene, a key indicator for the progression from early cognitive changes to more advanced stages of Azlheimer’s disease. Additionally, the paper evaluates the power of cutting-edge diagnostic tools, namely Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI). These non-invasive techniques are crucial for spotting the early pathological signs of AD well before clinical symptoms appear, offering a window for timely intervention.
Key Term Table
- Alzheimer’s disease (AD): A degenerative brain disease that falls under the category of dementia, caused by complex brain changes following cell damage
- Amyloid beta: A large membrane protein that plays an essential role in neural growth and repair. However, when it malfunctions it can lead to Alzheimer’s disease.
- Apoptosis: A form of programmed cell death
- Atrophy: Decreasing in size of body tissue
- Blood Brain Barrier (BBB):A network of blood vessels and tissue that helps keep harmful substances from reaching the brain
- Cerebrospinal fluid: A clear fluid found within the tissue surrounding all vertebrates’ brains and spinal cords. It helps cushion and provide nutrients.
- Dementia: A general term for several diseases affecting memory, thinking, and the ability to perform daily activities.
- Endothelial cells: A thin layer of cells that line the interior surface of blood vessels, forming a barrier between the bloodstream and the body’s tissues.
- Mild cognitive impairment (MCI): A condition in which people experience a slight but noticeable decline in memory or thinking skills, more than expected with normal aging.
- SCD: When a person feels they have a decline in memory or cognition, but it’s not detectable on standard memory tests.
- Tau: Tau is a naturally occurring protein that helps stabilize the internal skeleton of nerve cells (neurons) in the brain. When it malfunctions, it can lead to Alzheimer’s disease.
1. Introduction:
1.1 What is Dementia and Alzheimer’s disease?
Dementia encompasses a range of symptoms associated with a decline in memory, thinking, and decision-making abilities, significantly impacting daily life activities. According to the World Health Organization, around 50 million people worldwide have dementia, and there are nearly 10 million new cases every year (Dementia, n.d.). It is not a single disease but rather a broad term that covers various underlying conditions, with Alzheimer’s disease being the most prevalent, accounting for 60 to 80 percent of all dementia cases (What Are Alzheimer’s Plaques and Tangles? | BrightFocus Foundation, n.d.).
Alzheimer’s is a degenerative brain disease that is caused by complex brain changes following cell damage. The symptoms of Alzheimer’s can vary from one person to another and gradually worsen over time. Some symptoms in its earliest stages include losing things, and forgetting dates. Symptoms in its most severe stages include inability to communicate, no awareness of recent experiences, and seizures (What Are the Signs of Alzheimer’s Disease?, n.d.). Research indicates that these brain alterations begin well before noticeable memory loss occurs, often a decade or more, during a phase when individuals appear symptom-free. “During this preclinical stage of Alzheimer’s disease, people seem to be symptom-free, but toxic changes are taking place in the brain,” according to the National Institute on Aging. This preclinical stage involves malfunctioning two naturally occurring proteins: beta-amyloid and tau (Alzheimer’s Disease: The Basics – , n.d.).
Abnormal levels of beta-amyloid build up in the brain and form plaques between nerve cells. While inside the nerve cells tau creates neurofibrillary tangles, or twisted fibers. It is thought that the beta-amyloid plaque buildup outside the neuron initiates pathways inside the neuron that, in consequence, cause these neurofibrillary tangles. These toxic protein build ups prevent the nerve cells, or neurons, from transmitting information as they otherwise would. They lose connections with other nerve cells and die over time due to apoptosis (Amyloid and Tau: The Proteins Involved in Dementia — DPUK, n.d.).
Alzheimer’s disease initially affects the hippocampus, the brain region essential for memory formation. As the disease progresses, other brain areas are also affected and shrink (Alzheimer’s Disease: Causes, Stages, Symptoms & Prevention, n.d.).
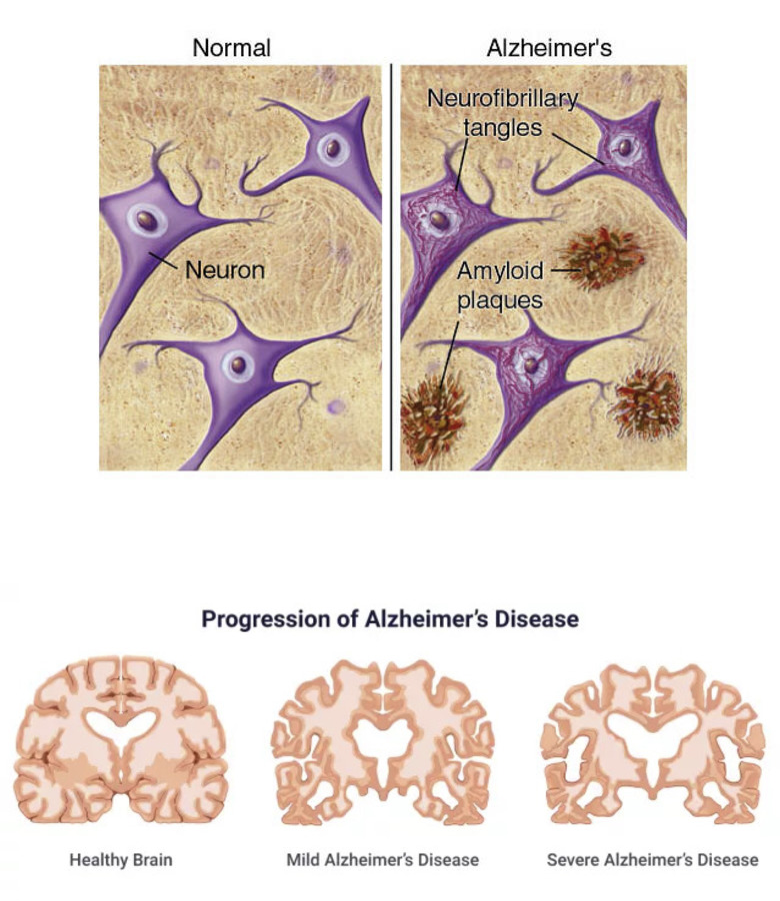
Sources : (What Are Alzheimer’s Plaques and Tangles? | BrightFocus Foundation, n.d.)
Figure 1: (A) Alzheimer’s disease develops amyloid plaques between neurons and neurofibrillary tangles inside neurons, preventing neurons from transmitting information.
(B) As Alzheimer’s disease progresses, the hippocampus is the first area to reduce its volume but then the other areas of the brain are also affected and begin to shrink. By the disease’s final stage, the damage is widespread, and brain tissue has radically shrunk.
1.2 What is Subjective Cognitive Decline?
Subjective Cognitive Decline (SCD) refers to individuals’ self-reported experiences of deteriorating memory or increased confusion. It is one of the initial signs of Alzheimer’s disease and related dementias that a person might notice. Some cognitive decline can occur as adults age, but frequently forgetting how to perform routine tasks is not a normal part of aging and can affect a person’s ability to live and function independently, which is SCD. However, SCD doesn’t equate to a clinical diagnosis of cognitive decline confirmed by a healthcare provider (life.1, n.d.)
Around 11.1% of adults in the US, or approximately 1 in 9, report experiencing SCD. SCD is less understood compared to Mild Cognitive Impairment (MCI) and Alzheimer’s disease (life.1, n.d.). Its clinical significance is often debated due to challenges in defining and diagnosing SCD in patients.
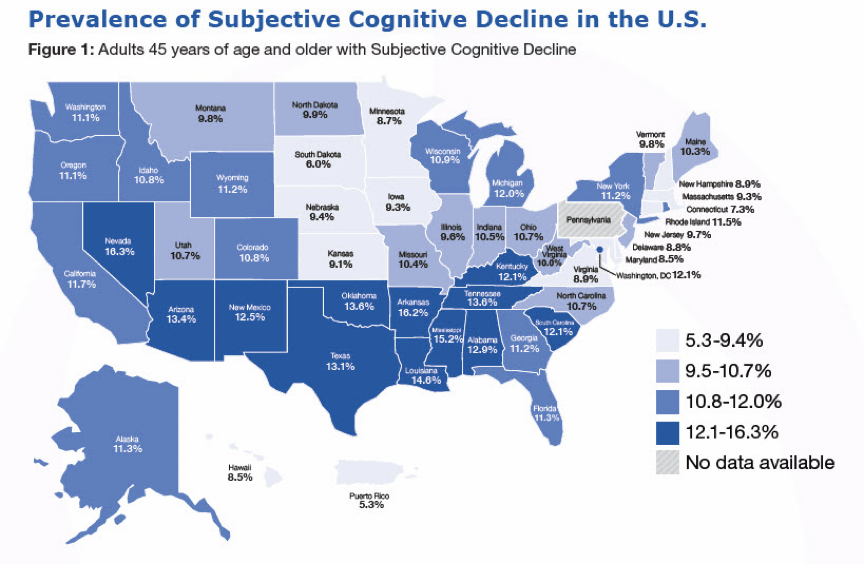
Figure 2: The map illustrates the state-by-state prevalence of Subjective Cognitive Decline (SCD) among Americans aged 45 and older, showcasing regional variations across the country. The shades of blue indicate the percentage of the population reporting SCD, with the darker tones representing higher prevalence. It highlights Nevada, Mississippi, Louisiana, Oklahoma, and Tennessee as the states with the highest percentage of reported cases of SCD,
1.3 What is Mild cognitive impairment?
Mild cognitive impairment (MCI) is when people have more memory or thinking problems than other people their age. The symptoms of MCI are not as severe as those of Alzheimer’s disease or related dementia but are considered to be more severe than SCD. In contrast with SCD, MCI is regarded as a clinical diagnosis and involves noticeable cognitive decline that is detectable through clinical evaluation and cognitive testing. Signs of MCI include losing things, forgetting to go to important events or appointments, and having more trouble coming up with words than other people of the same age. People with MCI can usually take care of themselves and carry out their normal daily activities, but it’s prevalent for family and friends to notice these changes. MCI is very common among the elderly; roughly 10% to 20% of people over age 65 have MCI, with the risk increasing as someone gets older (What Is Mild Cognitive Impairment?, n.d.).
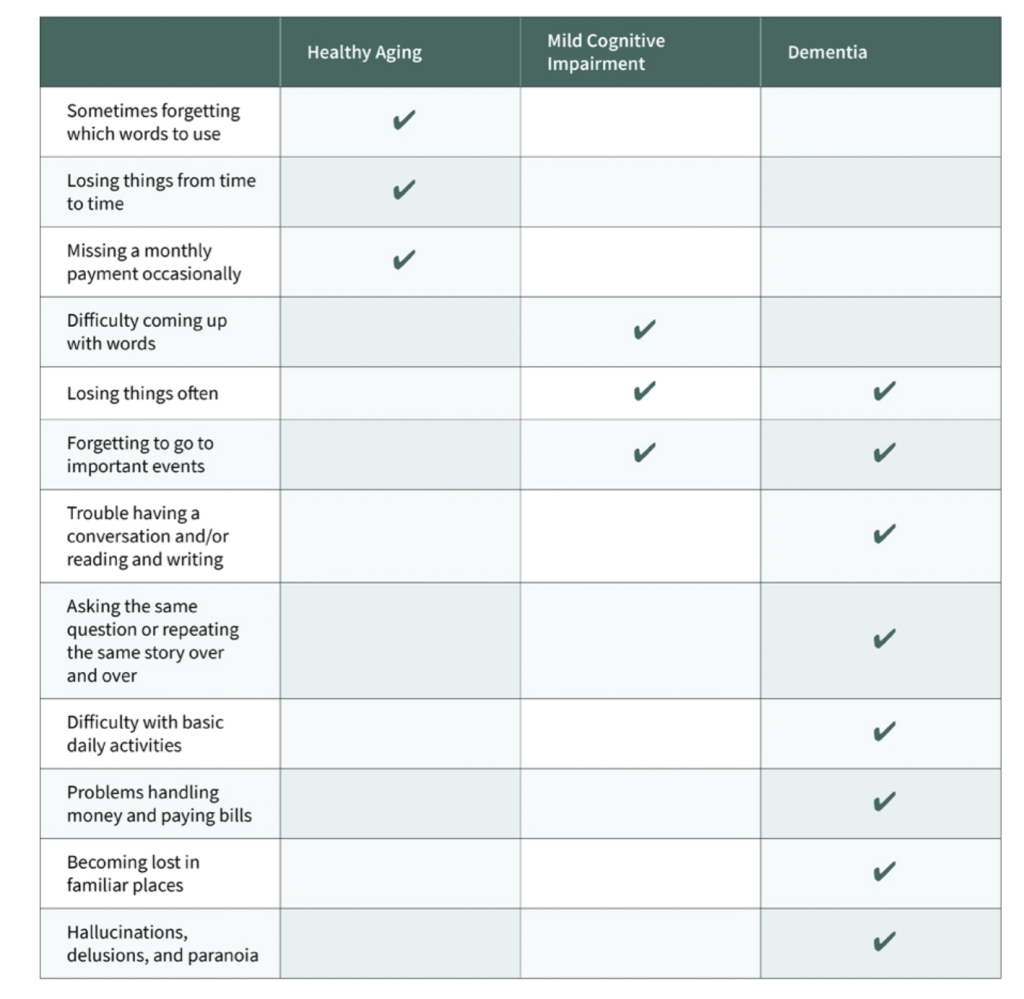
Table 1: This chart provides a detailed comparison of how Mild Cognitive Impairment (MCI) symptoms differ from those experienced by an elderly person in good health versus a patient with dementia.
2. Causes of Alzheimer’s disease:
Although the exact causes of Alzheimer’s Disease remain unknown, increasing evidence suggests that environmental and lifestyle factors, including exposure to pollutants and chemical waste, may play a significant role. Research, including systematic reviews and meta-analyses, consistently points to a link between exposure to pesticides and a heightened risk of developing AD. For example, a study in 2019 found that individuals exposed to pesticides had at least a 50% higher risk of AD than those not (Elonheimo et al., 2021).
In addition to pesticides, exposure to certain heavy metals, namely arsenic, mercury, and cadmium, has also been linked to an increased risk of AD. A study in 2018 demonstrated that AD patients had significantly higher levels of mercury and cadmium in their systems than healthy controls, indicating that these toxic metals might contribute to AD’s progression (Elonheimo et al., 2021).
| 2.1 Box 1: The Blood Brain Barrier (BBB) The brain, a uniquely complex organ, stands out for its sensitivity and the necessity for a controlled environment to ensure optimal functionality. This distinctiveness is underscored by the blood-brain barrier (BBB), a highly selective permeability barrier that plays a crucial role in maintaining cerebral health. The BBB functions as a natural protective membrane that carefully balances the passage of substances between the bloodstream and the central nervous system (CNS). It does so through microvascular endothelial cells (BMVEC), which shield the brain from potentially toxic substances and facilitate the supply of essential nutrients to brain tissues and remove harmful compounds back into the bloodstream (Daneman & Prat, 2015). While the BBB is essential for protecting the brain from toxins and pathogens, it introduces significant challenges in the study and treatment of neurological disorders, including Alzheimer’s disease. The BBB’s selective permeability complicates the delivery of pharmacotherapies because it impedes most chemical drugs and biopharmaceuticals from entering the brain. This restriction leads to low therapeutic efficacy in treating conditions like Alzheimer’s, as the drugs cannot easily reach their target areas within the brain. |
2.3 Connections of SCD and MCI with Dementia and Importance of Early Detection:
The early identification of Subjective Cognitive Decline (SCD) and Mild Cognitive Impairment (MCI) plays a critical role in the clinical research, prevention, and management of Alzheimer’s disease. The progression typically begins with SCD, advances to MCI, and eventually leads to dementia.
SCD is recognized as a significant risk factor for dementia, manifesting approximately 15 years before the onset of MCI (Shim et al., 2022). A study done by Dementia and Neuropsychologia showed that subjects with SCD exhibited a significantly elevated risk for the development of cognitive impairments when compared to their counterparts without SCD. Their data showed there was a 7.23% cumulative risk of transition from SCD to dementia across an average monitoring duration of 5.27 years (Parfenov et al., 2020). Individuals diagnosed with MCI face an increased likelihood of developing Alzheimer’s disease or related dementias. Estimates suggest that approximately 10% to 20% of individuals aged 65 or older with MCI may develop dementia within a year (What Is Mild Cognitive Impairment?, n.d.).
The emergence of disease-modifying drugs aimed at slowing the onset and progression of AD dementia has been met with challenges, primarily due to the timing of treatment initiation. Most treatments have been initiated too late after significant brain tissue injury has already occurred in AD. This observation underlines the critical need for initiating AD-related clinical studies and interventions during the preclinical stages. In this way, SCD and MCI are critical windows for potentially more effective intervention (Shim et al., 2022).
2.4 Genomic Predictors of Alzheimer’s Disease Progression
A metadata study by Sonia Moreno Grau and Agustin Ruiz highlights the crucial role of specific proteins and genomic markers in predicting the transition from SCD and MCI to Alzheimer’s disease, offering insights into potential avenues for early detection.
The risk of AD dementia is influenced by a broader genomic landscape, with research to identify 28 genes associated with the onset of Alzheimer’s Disease (LOAD). Among these, APOE stands out as a confirmed indicating factor in the transition from MCI to AD. Conversely, the relevance of APOE diminishes in the context of SCD progressing to Alzheimer’s disease. This observation aligns with the understanding that SCD represents an earlier stage of cognitive decline preceding MCI. Therefore, the APOE protein may not be as prevalent or influential during this initial phase (Genome Research in Pre-Dementia Stages of Alzheimer’s Disease – , n.d.).
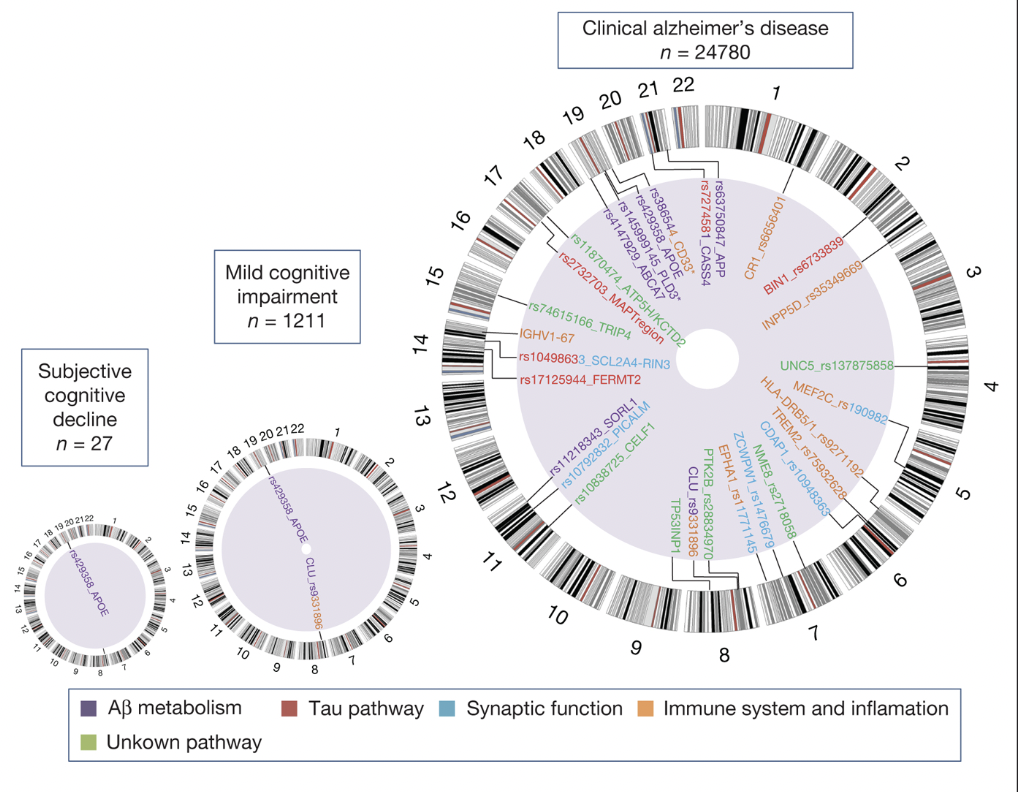
Figure 3: The figure shows the genes associated with subjective cognitive decline, mild cognitive impairment (MCI), and clinical Alzheimer’s disease, showing the APOE protein as a present gene in all three stages (this does not measure the risk of transition from one stage to another). It also represents the level of genomic information at each state. As one progresses from SCD to MCI and then to Alzheimer’s disease, there is an increase in the number and diversity of genomic associations, corresponding to the increasing severity and symptom complexity of the disease. The presence of multiple pathways in the Alzheimer’s disease stage indicates the multifactorial nature of the disease involving various biological processes.
3. The Role of Diagnostic Imaging in the Early Detection of AD
Numerous studies have highlighted that a significant portion of older adults with Subjective Cognitive Decline (SCD) exhibit biomarker abnormalities that align with those found in Alzheimer’s disease (AD). This has spurred interest in identifying reliable biomarkers for early detection. Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) are two diagnostic tools proven to effectively identify these AD-related biomarkers in individuals with SCD. Specifically, these biomarkers include increased amyloid uptake observed in PET scans, and medial temporal atrophy detected through MRI (Shim et al., 2022).
3.1 MRI scans of MCI and Alzheimer’s Disease
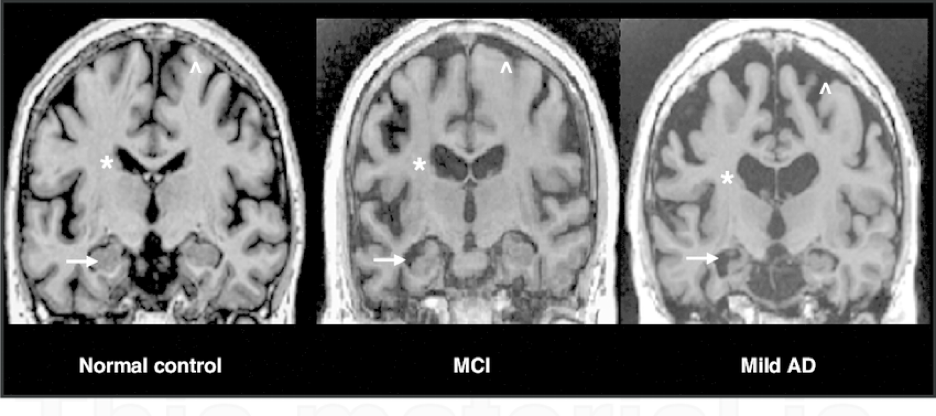
Figure 6: These MRI scans show the scan of a healthy brain on the left, a brain with mild cognitive impairment in the middle, and a brain with Alzheimer’s disease on the right. Alzheimer’s disease leads to decreased volume in the hippocampus, as shown with the arrows, and a ventricle enlargement, as labeled with the asterisk (labeled by the star). There is also shrinkage in the cerebral cortex, which is the brain’s outermost layer, causing it to shrivel up (labeled by the arrow pointing up).
3.2 PET Scans of Alzheimer’s Disease
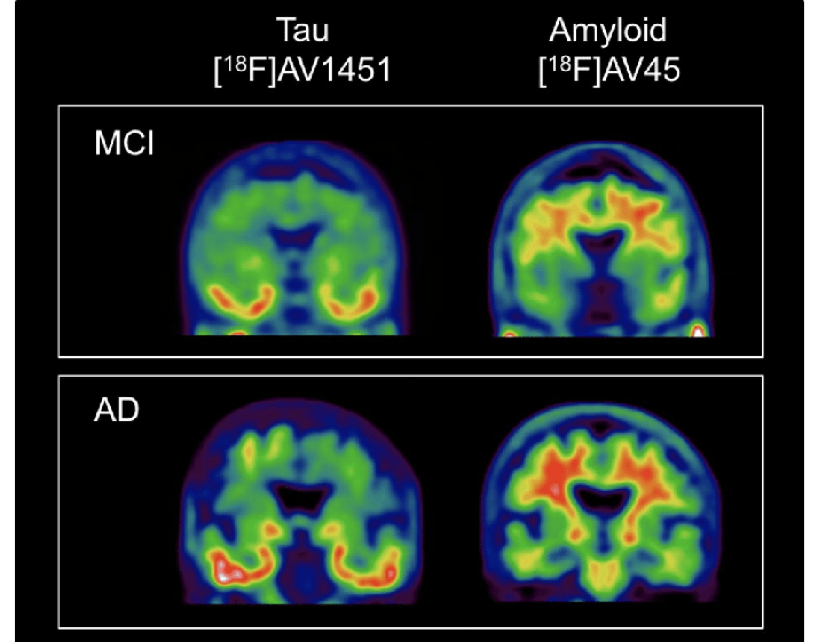
Figure 7: Shows PET scans of people with MCI and Alzheimer’s disease. The tau buildup is found in the temporal cortices (the parts of the brain that affect memory and understanding) and amyloid protein all over the brain but mainly in the frontal cortex and hippocampus. The scans clearly show how the buildup of tau and amyloid gets progressively worse in Alzheimer’s disease compared to MCI. A key idea of tau and amyloid deposits is that it’s not the amount but the location in the brain where they occur.
3.3 Comparative Analysis of Alzheimer’s Disease Detection Methods: PET, MRI, EEG, and Lumbar Puncture
PET scans stand out in detecting Alzheimer’s disease because they can visualize amyloid plaques using amyloid imaging agents. This capability stems from PET’s molecular imaging approach, which highlights the presence of amyloid plaques and provides functional information about the brain’s metabolic activity done at low reactivity. In Alzheimer’s disease, specific brain regions exhibit reduced metabolism early, which PET scans can effectively detect. Despite its high diagnostic value, one of the main limitations of PET scanning is its cost, which is much higher compared to other diagnostic methods (Zhang et al., 2016).
On the other hand, MRI is highly valued for its ability to produce high-resolution images of the brain’s structure, making it a viable option for detecting Alzheimer’s disease. The technique is beneficial for observing the brain’s hippocampus region, which typically undergoes atrophy in Alzheimer’s disease. MRI’s strength lies in its capacity to detect changes in brain volume and structure as the disease progresses. However, these structural changes usually manifest later in the disease course, which might make MRI less effective for early diagnosis (Zhang et al., 2016).
PET and MRI are both modern imaging techniques that are both in vivo and non-invasive. Lumbar puncture is a traditional method for detecting Alzheimer’s disease. This procedure is more invasive, involving the collection of cerebrospinal fluid (CSF) from the lower part of the spinal canal to analyze for Alzheimer’s biomarkers, such as abnormal levels of beta-amyloid or tau proteins. Although effective in identifying these biomarkers, the procedure can be uncomfortable for the patient and carries potential risks, including headache, infection, bleeding, or, in some cases, spinal cord damage (The Use of Lumbar Puncture and Safety Recommendations in Alzheimer’s Disease: A Plain Language Summary – , n.d.).
4. Discussion and Conclusion
The early detection and management of Alzheimer’s disease (AD) presents a complex challenge to the medical and scientific community, underscored by the multifaceted nature of the disease. Alzheimer’s disease, often considered to have various underlying conditions rather than a single entity, embodies a spectrum of neurodegenerative processes that contribute to its heterogeneity. This complexity reflects the diverse clinical manifestations and trajectories observed among patients and highlights the intricate interplay of genomic, molecular, and environmental factors that drive the disease’s progression.
The variability in the progression and onset of Alzheimer’s underscores the necessity for robust diagnostic tools capable of identifying the disease in its earliest stages. Non-invasive in vivo medical imaging techniques such as Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) have shown considerable promise, offering a window into the brain’s functional and structural changes long before clinical symptoms become apparent. The ability of PET scans to detect amyloid plaques and tau pathology, alongside MRI’s capacity to visualize structural brain alterations such as hippocampal atrophy, represents a pivotal advance in our approach to Alzheimer’s diagnosis and monitoring.
However, the challenge extends beyond the mere detection of AD biomarkers. The goal is to integrate these findings into a coherent framework that allows for the early identification of individuals at risk and the implementation of preventive or mitigating strategies. In this context, the advent of artificial intelligence (AI) in the analysis of PET and MRI could be a new era in Alzheimer’s research. AI algorithms, with their ability to discern patterns and anomalies based on vast datasets, offer the potential to enhance the diagnostic accuracy of imaging studies. By facilitating the early detection of AD-related changes and predicting disease progression, AI-driven tools can enable more timely and targeted interventions, potentially altering the disease’s trajectory.
Another promising approach is the area of personalized medicine, due to the variability and complexity of Alzheimer’s disease in each of its patients. Personalized medicine is rooted in the belief that since individuals have unique characteristics at the molecular, physiological, environmental exposure and behavioral levels, they need to have interventions provided to them for diseases they possess that are fit to these unique characteristics. This method allows the ability to predict which medical treatments will be safe and effective for each patient, and which ones will not be (Goetz & Schork, 2018).
Additionally, classifying Alzheimer’s disease into subtypes, including amyloid pathology, tauopathy, inflammatory, and cerebrovascular subtypes, represents a promising advancement towards addressing its complexity. This classification facilitates a deeper understanding of the disease and opens pathways to more specific and effective treatment approaches for each subtype, highlighting the crucial role of personalized interventions in the future of Alzheimer’s management
Works Cited
Alzheimer’s Disease: Causes, Stages, Symptoms & Prevention. (n.d.). Drugwatch.Com. Retrieved March 9, 2024, from www.drugwatch.com/health/alzheimers-disease/
Alzheimer’s Disease: The Basics – . (n.d.). Alzheimer’s San Diego. Retrieved March 9, 2024, from www.alzsd.org/resources/what-is-alzheimers-disease/
Amyloid and tau: the proteins involved in dementia — DPUK. (n.d.). Retrieved March 9, 2024, from www.dementiasplatform.uk/news-and-media/blog/amyloid-and-tau-the-proteins-involved-in-dementia
Daneman, R., & Prat, A. (2015, January 1). The Blood–Brain Barrier. PubMed Central (PMC); Cold Spring Harbor Laboratory Press. www.ncbi.nlm.nih.gov/pmc/articles/PMC4292164/
Dementia. (n.d.). Retrieved March 16, 2024, from https://www.who.int/news-room/fact-sheets/detail/dementia
Elonheimo, H. M., Andersen, H. R., Katsonouri, A., & Tolonen, H. (2021, November 1). Environmental Substances Associated with Alzheimer’s Disease—A Scoping Review. PubMed Central (PMC); Multidisciplinary Digital Publishing Institute (MDPI). www.ncbi.nlm.nih.gov/pmc/articles/PMC8622417/
Fig. 1 PET with [ 18 F]AV1451 and [ 18 F]AV45 showing differentiate… (n.d.). ResearchGate. Retrieved March 16, 2024, from www.researchgate.net/figure/PET-with-18-FAV1451-and-18-FAV45-showing-differentiate-uptake-patterns-of-tau-and_fig1_331099089
Fig. 4. Structural MRI of control, MCI, and mild AD individuals,… (n.d.). ResearchGate. Retrieved March 10, 2024, from www.researchgate.net/figure/Structural-MRI-of-control-MCI-and-mild-AD-individuals-showing-mild-MCI-and_fig1_51904872
Genome research in pre-dementia stages of Alzheimer’s disease – . (n.d.). PubMed. Retrieved March 10, 2024, from pubmed.ncbi.nlm.nih.gov/27237222/
Goetz, L. H., & Schork, N. J. (2018, June 1). Personalized Medicine: Motivation, Challenges and Progress. PubMed Central (PMC); NIH Public Access. www.ncbi.nlm.nih.gov/pmc/articles/PMC6366451/life.1. (n.d.). Subjective Cognitive Decline — A Public Health Issue.
Parfenov, V. A., Zakharov, V. V., Kabaeva, A. R., & Vakhnina, N. V. (2020, September 1). Subjective cognitive decline as a predictor of future cognitive decline: a systematic review. PubMed Central (PMC); Academia Brasileira de Neurologia. www.ncbi.nlm.nih.gov/pmc/articles/PMC7500809/
Shim, Y., Yang, D. W., Ho, S., Hong, Y. J., Jeong, J. H., Park, K. H., Kim, S., Wang, M. J., Choi, S. H., & Kang, S. W. (2022, October 1). Electroencephalography for Early Detection of Alzheimer’s Disease in Subjective Cognitive Decline. PubMed Central (PMC); Korean Dementia Association. www.ncbi.nlm.nih.gov/pmc/articles/PMC9644061/
The use of lumbar puncture and safety recommendations in Alzheimer’s disease: a plain language summary – . (n.d.). PubMed. Retrieved March 30, 2024, from pubmed.ncbi.nlm.nih.gov/35866715/
What are Alzheimer’s Plaques and Tangles? | BrightFocus Foundation. (n.d.). Retrieved March 9, 2024, from www.brightfocus.org/news/amyloid-plaques-and-neurofibrillary-tangles
What Are the Signs of Alzheimer’s Disease? (n.d.). National Institute on Aging. Retrieved March 16, 2024, from www.nia.nih.gov/health/alzheimers-symptoms-and-diagnosis/what-are-signs-alzheimers-disease
What Is Mild Cognitive Impairment? (n.d.). Retrieved March 10, 2024, from www.alzheimers.gov/alzheimers-dementias/mild-cognitive-impairment
Zhang, X. Y., Yang, Z. L., Lu, G. M., Yang, G. F., & Zhang, L. J. (2016, December 31). PET/MR Imaging: New Frontier in Alzheimer’s Disease and Other Dementias. PubMed Central (PMC); Frontiers Media SA. www.ncbi.nlm.nih.gov/pmc/articles/PMC5672108/
About the author

Sofia Sattler
Sofia is currently a high school student interested in medicine, particularly neurodegenerative diseases. She has previously published research on Parkinson’s disease. In her free time, Sofia volunteers at dog shelters and enjoys playing tennis.