
Author: Jiajun Li
Mentor: Dr. Nageh K. Allam & Dr. Ali Ayoub
St. Andrew’s College
Abstract
With more industrial developments, there is an increased demand for clean energy. One source that can provide clean energy is hydrogen-based fuels. In the paper, the term “hydrogen power” means all energy generation methods based on hydrogen, such as hydrogen combustion or hydrogen fuel cells. However, generating hydrogen at an industrial scale requires scaling up hydrogen generation processes such as electrolysis. This depends on selecting suitable catalysts to expedite the process. This paper provides a review of existing theories that help identify potential catalysts for this process. Specifically, d-band theory and spinel theory predict the activity descriptors for Oxygen Evolution Reactions and Hydrogen Evolution Reactions, respectively.
Keywords: water electrolysis, hydrogen evolution reaction, oxygen evolution reaction, catalyst, d-band theory, spinel theory
The current world and social structure depend on generating electricity. There are various approaches to generating electricity, with the main options being using fossil fuels (combustion), renewable energy, and nuclear energy. In most fossil fuel generators, byproducts, mainly in the form of greenhouse gases (GHGs), will be produced due to the combustion reaction, and GHGs are capable of warming up the Earth’s climate and polluting the atmosphere (Markandya & Wilkinson, 2007, p. 979). According to data collected by a research team that published the findings in the journal “Earth System and Scientific Data,” a rigorous academic journal with very transparent processes, which are then compiled by Climate Watch, an organization under the World Resource Institute, the annual GHG emissions from the entire world, measured in billion tons of CO2 equivalent, from 1850 to 2016, it increased from 1.4373 billion tons to 46.50 billion tons or approximately a 3135 % increase in emissions. Most of these emissions come from energy demands (World Resources Institute, 2022). According to the same source, about 33% of the world’s emissions in 2021 came from electricity and heating, the largest sector of global GHG emissions (World Resources Institute, 2022). The data shows that energy production is a considerable portion of the global GHG emissions. Thus, a clear and most impactful solution to climate change will be finding a clean or low-carbon energy source, as it will directly address 30% of global emissions.
There are many ways to produce clean and non-polluting energy, such as solar energy, which is generated directly from sunlight. However, these methods are not perfect. Most renewable energy sources, particularly solar energy, are intermittent or unstable, requiring additional infrastructure to account for the problem (Mathew, 2022, p. 5). This, combined with the lack of a large and powerful energy storage system, leads to grids with renewable sources having to depend on fossil fuels, creating additional GHG emissions (Mathew, 2022, p. 5). Additionally, these renewable energy sources consume many resources, particularly land. For instance, a 1000 MW fossil fuel power plant requires 1-4 km2 of land for the entire facility, while renewables require a lot more land, with solar requiring 20-50 km2, wind requiring 50-150 km2 , and biomass requiring 4000-6000 km2 (Rashad & Hammad, 2000, p. 213). These factors combined make most of the current renewable energy systems unable to generate electricity as effectively as methods like fossil fuel. They could potentially release additional GHGs from the extra land use and infrastructure. However, not all renewable energy sources have that problem, and using hydrogen power can prevent these problems.
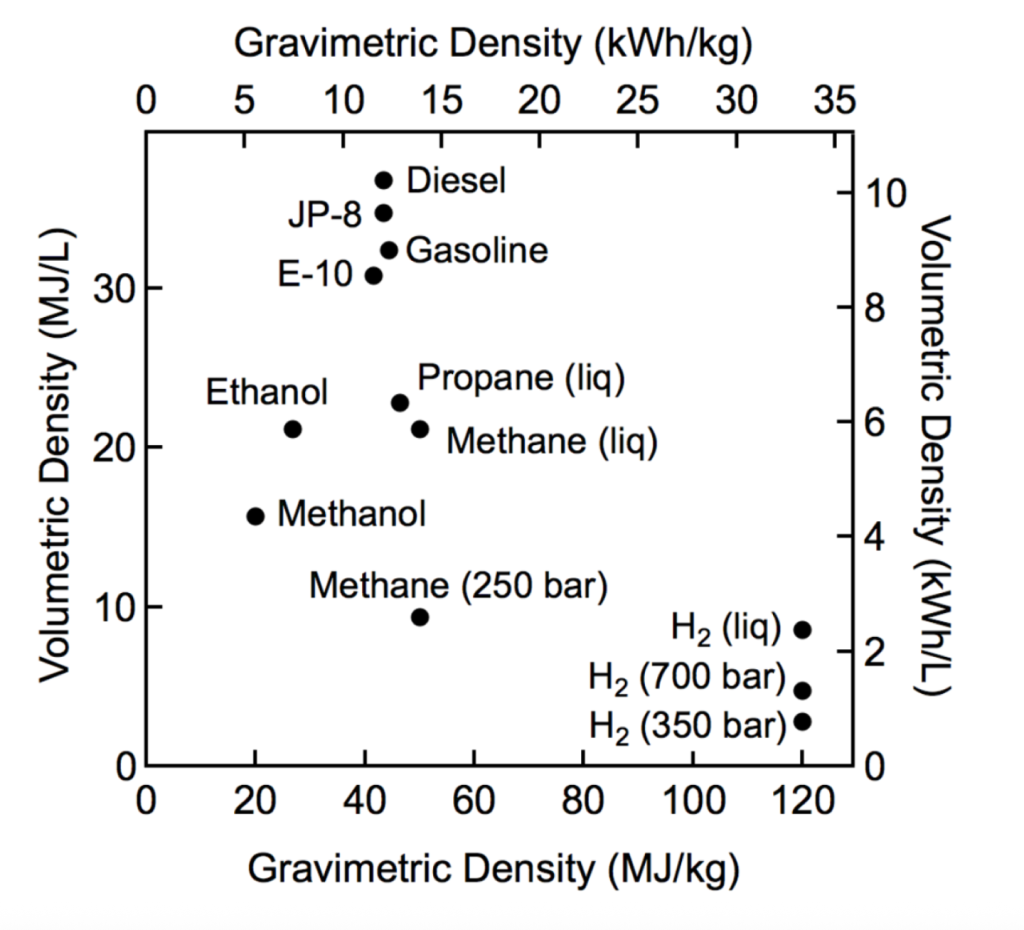
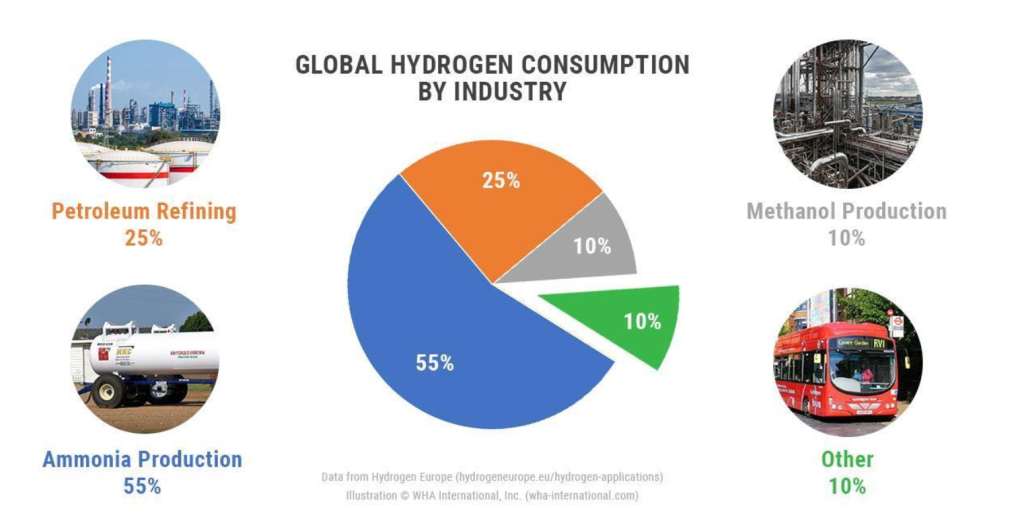
Hydrogen has many advantages as an element in itself. It is a highly energy-dense element (in terms of mass), making it comparable with other standard energy production methods, such as fossil fuels like petroleum or coal, as shown in Figure 1 (U.S. Department of Energy, n.d.-b). Hydrogen power has an effective energy system that is proven by fossil fuels. The underlying principle of hydrogen power is the same as that of fossil fuels, converting hydrogen combustion’s thermal energy to steam’s kinetic energy by boiling water, finally pushing a turbine with that kinetic energy, and generating electricity. This process has been proven by decades of application and is widely used today. Approximately 42% of all electricity generation in the United States uses steam turbines (U.S. Energy Information Administration, 2023). Hydrogen also comes with the added benefit of not producing any pollutants when burned, with its byproduct being only water, which is the product of the hydrogen combustion reaction. Not only that, but hydrogen can also be used in fuel cells, which is another way to produce power efficiently, with its efficiency ranging from 40% to 60% (U.S. Department of Energy Energy Efficiency & Renewable Energy, 2010). Hydrogen is also an essential industrial element, as shown in Figure 2, commonly used in industries like agriculture, where it can synthesize ammonia, a key component in all modern fertilizers (WHA International Inc, 2023; World Nuclear Association, 2024).
Background and Literature Review
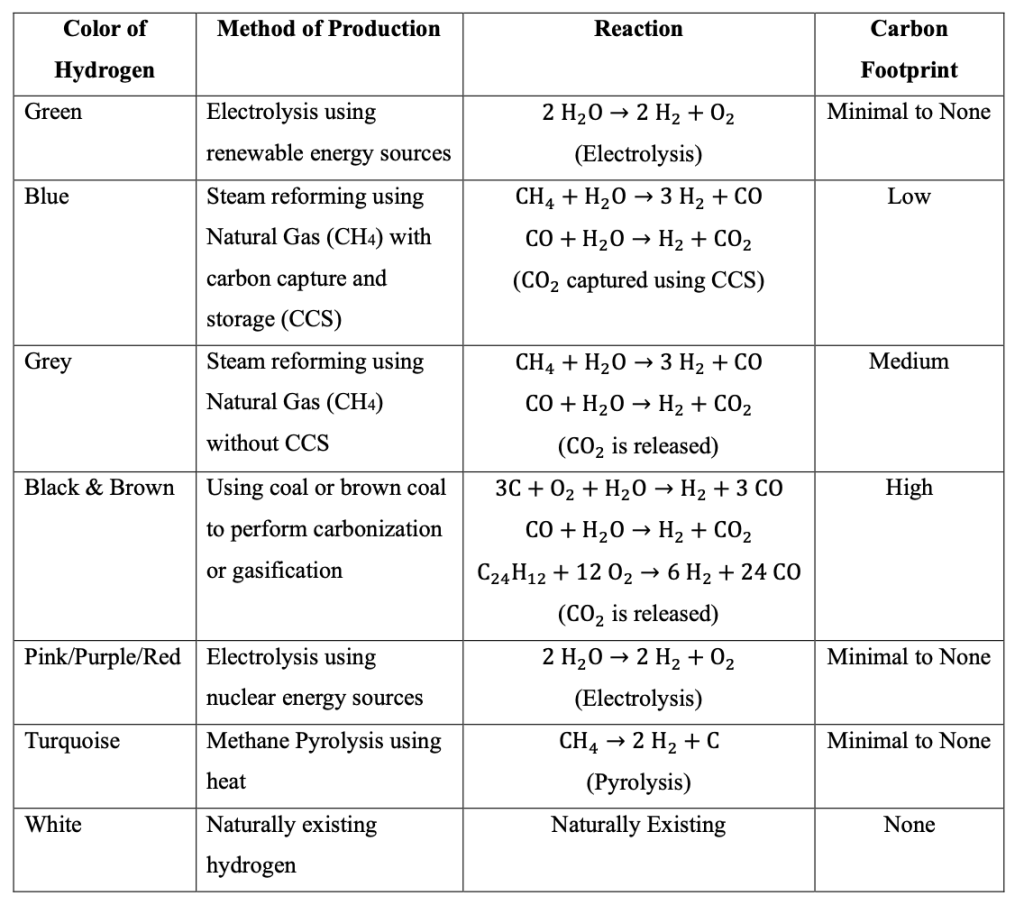
Despite the benefits of a hydrogen-based energy system, getting hydrogen clean is a challenge. There are various ways to produce hydrogen; however, most are produced using fossil fuels, which release GHGs. In fact, approximately 95% of the world’s hydrogen production is based on fossil fuels and releases GHGs (Rosenow, 2022). In the case of hydrogen power, generating power with hydrogen that has a considerable amount of carbon footprint attached to it during its production process will make the purpose of hydrogen power obsolete, and thus, using renewable or clean hydrogen is essential to ensuring the benefits of hydrogen power can be released at full potential. To better classify different types of hydrogens, color codes are assigned to them, with different colors representing different carbon footprint levels, as shown in Table 1 (National Grid, 2025). Based on the classification of hydrogen, for hydrogen power to be completely carbon-free, using green hydrogen is the best approach. Electrolysis is essential to creating green hydrogen, as detailed in Table 1. It works by splitting water molecules, which consist of two hydrogen atoms and one oxygen atom. Thus, running a specific voltage through the water molecules will break the chemical bond between the atoms and release the atoms themselves. This method is carbon neutral and does not release additional GHGs, assuming the electricity used for the electrolysis is carbon neutral.
In an electrolysis reaction, the electric current passed through serves as the activation energy of the reaction to dissociate water molecules into hydrogen and oxygen. This happens because when an electrical current is passed through the anode, cathode, and the water itself, the water molecules undergo oxidation at the anode, producing oxygen gas and releasing electrons. At the same time, the hydrogen from the oxidation is also reduced at the cathode, where hydrogen ions are being reduced by gaining an electron from the oxidation at the anode, finally creating both hydrogen and oxygen gas at the ends. This process, however, is very energy- intensive as it needs to overcome a strong energy barrier presented by the OH bonds in water. These bonds in water have an average bond energy of 461.5 kJ/mol, an accepted value, which is quite strong (Song & Le, 2013). As a result, for the reaction to occur, more energy has to be passed through, making the process less efficient and more challenging to complete, decreasing the possibility for it to be used in large-scale industrial processes such as generating hydrogen in large enough quantities to supply power plants without a method to decrease the amount of energy used.
As a result, catalysts are being used to lower the amount of energy needed in this process, as a catalyst can lower the activation energy of reactions while not consuming itself during the reaction (U.S. Department of Energy, n.d.-a). This can be used to boost the amount of hydrogen acquired from electricity, improving the efficiency of the electrolysis reaction. There are various types of catalysts with pros and cons, as well as having properties that are more inclined to support either the oxidation or the reduction reaction. In the industry, the key to successfully creating and commercializing the system for broad public use is to find a suitable catalyst that balances various qualities. This can be done by reliably identifying the activity descriptor, which represents a quantifiable indicator for a catalyst’s capability to catalyze a specific reaction, in this case, the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). Thus, this paper focuses on reliably identifying the activity descriptors for the hydrogen and oxygen evolution in the water electrolysis reaction.
HER and OER
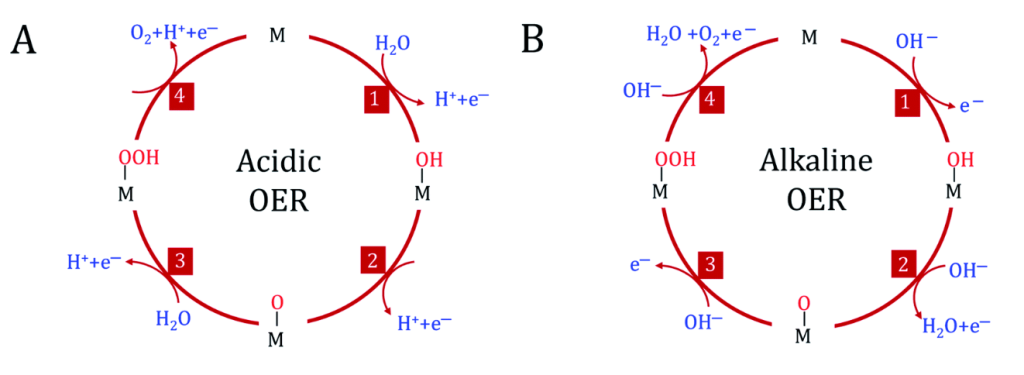
As established before, the HER reduces hydrogen ions, and the OER oxidizes water molecules to release oxygen. Generally speaking, the OER is more energy-intensive and slower than the HER because it has a more complex reaction mechanism. Specifically, there are four electron transfer processes during the OER, as shown in Figure 3. This multi-step process requires breaking the strong OH bond in water to generate oxygen. The four-electron transfer process also means that forming multiple intermediates is challenging (J. Li, 2022). This, in turn, creates kinetic barriers, making it much slower and requiring a higher overpotential (extra energy) to make the reaction happen. On the other hand, the HER only requires 2 electron transfers, meaning it is essentially a more straightforward process that is also easier to achieve comparatively (Dubouis & Grimaud, 2019).
Additionally, during the OER, various intermediates containing oxygen form on the catalyst and get absorbed onto its surface. The formation of these intermediates is a critical step in the process, as this is the only way catalysts can facilitate the breaking and formation of oxygen molecules, which are the intended product. However, this process is challenging to balance as too much binding force will slow down the overall kinetics of the reaction, and the reaction could become “stuck” (J. Li, 2022). This is not a problem for HER because hydrogen atoms and their intermediates are much smaller, easier to release from the catalyst, and involve fewer steps to convert to their molecular form of H2 (Dubouis & Grimaud, 2019).
d-band Theory and Catalyst Performance Prediction for OER
The d-band theory is a fundamental concept used to explain and predict the performance of a transition metal catalyst in a reaction, where it applies specifically to transition metals because of the filled d-orbitals (Bhattacharjee et al., 2016). The theory mainly revolves around the d-band center, which is the average energy level of electrons in the d orbital relative to the Fermi level and is the highest possible energy for electrons at absolute zero, which serves a crucial role in catalytic activity (Bhattacharjee et al., 2016).
The theory’s predictions depend on the relative position of the d-band center (Bhattacharjee et al., 2016). Regarding OER specifically, the theory can predict how well a catalyst binds with oxygen-containing intermediates such as OH, O2, and OOH. When the d- band center is closer to the Fermi level, the interaction between the catalyst and the reaction intermediates generally increases. Conversely, a larger proximity will weaken these interactions (Bhattacharjee et al., 2016). This does not mean that aiming for the highest d-band center (i.e., closest proximity) will be the best. As explained above, a too-strong interaction will hinder oxygen molecules’ release (desorption) and will have a lower efficiency overall. Using the same logic, if the d-band center is too low, the intermediates will not bind strongly, leading to a higher activation energy requirement for the reaction (Bhattacharjee et al., 2016). As a result, catalysts with an optimal d-band center are defined as those that can strike a good balance between adsorption and desorption, allowing for an efficient intermediate process without energy losses, meaning higher overall efficiency. This is because the optimal balance of the d-band center can minimize overpotentials, which will enhance catalytic performance by wasting less energy, leading to a higher energy efficiency, thus allowing more energy to be applied to the reaction itself, increasing the reaction rate (Bhattacharjee et al., 2016). Additionally, over-potential has side effects like releasing heat and increasing material/catalyst stress, which can lead to a shortened lifetime and be suboptimal for future industrial operations. On the other hand, a lower overpotential will help maintain the stability of the catalyst, reducing wear and tear and, as a result, prolonging its lifespan, ultimately leading to the development of cheap and practical catalysts (Dubouis & Grimaud, 2019).
Application of d-band Theory in Catalyst Manufacturing and Design
The d-band theory can be applied in catalyst designs and manufacturing, with it serving as the guiding principle for tailoring catalysts by adjusting the electronic structure via alloying or doping various materials (Chen & Zhang, 2022). The theory has been validated through Density Functional Theory (DFT) calculations, a standard tool for validating and studying catalysts, supporting the effectiveness of the d-band theory (Nørskov et al., 2011). As a result, the d-band Theory can be used as a foundational principle for designing more advanced catalysts that work best for water electrolysis because the d-band theory can enable researchers to have something tangible that they can change for different results (Chen & Zhang, 2022).
Limitations of the d-band Theory for Catalyst Design
Nevertheless, the d-band theory has downsides as it does not apply universally to all catalyst materials (Bhattacharjee et al., 2016). As different catalysts have different electronic structures and surface morphologies, these factors lead to different catalysts requiring a different descriptor that considers and optimizes these factors (B. Wang & Zhang, 2022). For instance, the d-band theory works because it is based on the d orbital electrons, which can play a significant role in reaction intermediates (Bhattacharjee et al., 2016). However, the theory cannot accurately predict the catalytic behavior for materials like metal oxides, on-transition metals, and complex systems, such as perovskites and platinum, because the materials’ structures are too complex to be oversimplified by the d-band behavior in d-band theory (Gorzkowski & Lewera, 2015; B. Wang & Zhang, 2022).
Brief Explanation of HER Mechanism
Moving on to the hydrogen side of the reaction. The HER is a crucial aspect of water electrolysis and the source of green hydrogen. However, despite being generally more straightforward regarding reaction mechanism, HER does not have a definitive or universal theoretical model to predict catalyst performance, unlike OER, which has the d-band theory (Zheng et al., 2018). This makes identifying the optimal catalyst for the reaction completely different and requires understanding and analyzing unique activity descriptors that are not universally applicable to HER. Thus, an overview of HER catalyst design theories is presented below.
Cation Distribution and Spinel Theory for HER Catalyst Design
For HER, a very promising approach in catalyst design is using Spinels. Spinels are a type of crystalline material with the general formula of AB2O4, where “A” and “B” represent different metal cations, and “O” represents oxygen (Elkholy et al., 2017). Spinels generally have a cubic crystal structure characterized by two potential types of sites where the cation can be situated: the Tetrahedral or A site and the Octahedral or B site (Elkholy et al., 2017). These materials are known for their robustness, high thermal stability, and electrical conductivity, making them ideal for industrial applications after an optimal catalyst based on Spinels is successfully developed (Elkholy et al., 2017). One example of spinel is CoFe2O4. In this case, the Co2+ ion occupies the tetrahedral (A) sites while the Fe3+ ion occupies the octahedral (B) sites, together with the four oxygen atoms forming the framework of the molecule (Gomaa et al., 2024).
As there are two sites where cations can reside, a balance needs to be reached between these cations, denoted by δ. The balance significantly affects the catalytic activity for HER, and research has demonstrated that catalysts with an optimal cation distribution can substantially improve catalytic performance. This is because there is a better electron transfer process for the reaction and a more optimized binding strength of the hydrogen intermediates similar to hydrogen (Gomaa et al., 2024). For instance, the spinel of CoFe2O4 is an optimal catalyst for HER. CoFe2O4 has a cation distribution of δ of 0.33, and further research shows that CoFe2O4 exhibits low overpotentials, as low as 66 mV, which is advantageous as low levels of overpotential generally translate to a higher reaction efficiency (Gomaa et al., 2024; Niu et al., 2020). The arrangement of cations in the sites will influence the electronic structure of the spinel, similar to the d-band theory but with much more complicated mechanics (Gomaa et al., 2024). This change in electronic structure will optimize the interaction with hydrogen intermediates, reaching the right balance of binding strength (Exner, 2022).
Hydrogen Adsorption and Desorption Energy for HER Catalyst Analysis and Design
In addition to cation distribution, hydrogen and hydroxyl ions (OH-) adsorption energy has a crucial role in HER, which is especially important in alkaline media with a higher concentration of hydroxyl ions. In a study conducted by Baghban and colleagues, they used DFT to calculate the adsorption energies and achieved a 96.7% accuracy on predicting the behavior of actual catalysts (2021). An ideal catalyst for HER will exhibit a Gibbs free energy close to zero for hydrogen adsorption, meaning it will need less and less energy for the reaction to happen, or, in other words, a lower activation energy given that the catalyst can also efficiently adsorb and dissociate water to provide hydrogen for the reaction (Hu et al., 2016).
Outlook for HER Catalyst Descriptor
Due to the unique nature of the HER, it is essential to consider the current outlook for catalyst design. There are promising developments for HER catalyst descriptor analysis, but a universal and consistently working descriptor theory for HER still does not exist (Dubouis & Grimaud, 2019). Unlike in the case of OER, HER cannot use d-band theory because of complications, and other theories suffer from the same problem, causing the lack of a consistently working universal descriptor theory for HER. The HER involves diverse electronic properties observed in materials available for HER, making it very challenging to establish a single definitive set of rules or descriptors that can apply universally (Du et al., 2025). Admittedly, the d-band theory cannot predict catalysts under all circumstances as previously established, but it is still a valuable OER catalyst analysis approach, which is “better” than the current HER situation. As such, future HER research should focus on developing a more comprehensive theory, allowing the community to progress towards a more comprehensive theory while enabling other potential research areas.
Conclusion
As established previously, energy is critical for society, so developing a clean energy source is also essential. However, current energy generation options have significant limitations, such as pollution or scalability. Specifically, despite being cheap and efficient, fossil fuels are very polluting, while on the other hand, despite being clean, solar power and other renewables are less efficient, intermittent, and consume large amounts of resources to create a working system (Rashad & Hammad, 2000). An alternative to all these methods exists: using hydrogen as a clean fuel source. Hydrogen is an excellent alternative to fossil fuel, as it has a high energy density and low emissions (Hossain Bhuiyan & Siddique, 2025). In addition to that, hydrogen also matters in other fields, as it is an essential industrial resource. The side product generated by green hydrogen production, oxygen, also has an essential industrial application, making green hydrogen production even more tempting (Eckl et al., 2025; U.S. Energy Information Agency, 2024).
Despite these benefits, hydrogen production is mainly achieved using fossil fuel (steam reforming), where 62% of all hydrogen production relies on natural gas (steam reforming), and around 99% of all hydrogen production requires fossil fuel and leads to carbon emissions (International Energy Agency, 2024). Therefore, when hydrogen is used in a hydrogen-based powerplant or a hydrogen fuel cell, it will likely have a carbon footprint comparable to that of normal fossil fuel. As such, developing a completely carbon-neutral method to produce hydrogen, specifically electrolysis, is essential. Nevertheless, electrolysis has problems because it is inefficient and not easily scalable, especially for industrial operations. To solve this issue, catalysts that can meet the requirements of an industrial system can be used to make the reaction less energy-consuming, hence allowing us to achieve efficient large-scale water electrolysis. This requires a theory that can reliably identify the respective activity descriptors for both the HER and OER. As discussed throughout the paper, the catalyst for the OER can be predicted using the d-band theory by optimizing the adsorption and desorption of oxygen-containing intermediates, whereas HER performance depends on more material-specific approaches and is generally harder to define. However, methods to identify the optimal HER catalysts exist, including understanding cation distribution in spinel systems that use catalysts of a specific format, such as CoFe2O4. These strategies demonstrate that by understanding electronic and atomic structures, specifically the d-band center in transition metal-based catalysts and spinel cation balance, the performance of catalysts for water electrolysis can be effectively and quantitatively predicted. In conclusion, optimizing catalyst selection and advancing in d-band and spinel theory or other potential theories are necessary for the bigger goal of large-scale clean hydrogen production. Therefore, more focus, funding, and research should be directed towards understanding and developing these catalysts to enable their industrial use, from energy production to industrial uses, not only laboratory applications.
This paper provides a review of the current available methods in identifying the activity descriptors for both the HER and OER and does not aim to find new methods or theories. To solve the problem of catalyst design, more experimental trials and data on catalyst designs need to be done, which will enable the potential for further understanding of catalysis or even potentially finding the catalyst that can be applied in the industry. Also, this review does not include all aspects of the research, as the paper only discussed the theories related to water electrolysis and their associated catalysts, which themselves can still benefit from catalysis research developments in other fields. Specifically, catalysis research has been done in fields other than water electrolysis catalysis, and we anticipate that future work could incorporate findings from those fields into the field of water electrolysis. Doing so can compile a more comprehensive and effective review, providing more value to the field.
References
Baghban, A., Habibzadeh, S., & Zokaee Ashtiani, F. (2021). On the evaluation of hydrogen evolution reaction performance of metal-nitrogen-doped carbon electrocatalysts using machine learning technique. Scientific Reports, 11(1), 21911. https://doi.org/10.1038/s41598-021-00031-0
Bhattacharjee, S., Waghmare, U. V., & Lee, S.-C. (2016). An improved d-band model of the catalytic activity of magnetic transition metal surfaces. Scientific Reports, 6(1), 35916. https://doi.org/10.1038/srep35916
Chen, Z., & Zhang, P. (2022). Electronic structure of single-atom alloys and its impact on the catalytic activities. ACS Omega, 7(2), 1585–1594. https://doi.org/10.1021/acsomega.1c06067
Du, J., Yan, Y., Li, X., Chen, J., Guo, C., Chen, Y., & Wang, H. (2025). A mechanism-guided descriptor for the hydrogen evolution reaction in 2D ordered double transition-metal carbide MXenes. Chemical Science (Royal Society of Chemistry: 2010), 16(21), 9424– 9435. https://doi.org/10.1039/d4sc08725a
Dubouis, N., & Grimaud, A. (2019). The hydrogen evolution reaction: from material to interfacial descriptors. Chemical Science, 10(40), 9165–9181. https://doi.org/10.1039/c9sc03831k
Eckl, F., Moita, A., Castro, R., & Neto, R. C. (2025). Valorization of the by-product oxygen from green hydrogen production: A review. Applied Energy, 378(124817), 124817. https://doi.org/10.1016/j.apenergy.2024.124817
Elkholy, A. E., El-Taib Heakal, F., & Allam, N. K. (2017). Nanostructured spinel manganese cobalt ferrite for high-performance supercapacitors. RSC Advances, 7(82), 51888–51895. https://doi.org/10.1039/c7ra11020k
Exner, K. S. (2022). On the optimum binding energy for the hydrogen evolution reaction: How do experiments contribute? Electrochemical Science Advances, 2(4). https://doi.org/10.1002/elsa.202100101
Gomaa, A. K., Zonkol, M. G., Khedr, G. E., & Allam, N. K. (2024). Cation distribution: a descriptor for hydrogen evolution electrocatalysis on transition-metal spinels. EES Catalysis, 2(6), 1293–1305. https://doi.org/10.1039/d4ey00121d
Gorzkowski, M. T., & Lewera, A. (2015). Probing the limits of d-band center theory: Electronic and electrocatalytic properties of pd-shell–pt-core nanoparticles. The Journal of Physical Chemistry. C, Nanomaterials and Interfaces, 119(32), 18389–18395. https://doi.org/10.1021/acs.jpcc.5b05302
Hossain Bhuiyan, M. M., & Siddique, Z. (2025). Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. International Journal of Hydrogen Energy, 102, 1026–1044. https://doi.org/10.1016/j.ijhydene.2025.01.033
Hu, G., Tang, Q., & Jiang, D.-E. (2016). CoP for hydrogen evolution: implications from hydrogen adsorption. Physical Chemistry Chemical Physics: PCCP, 18(34), 23864–23871. https://doi.org/10.1039/c6cp04011j
International Energy Agency. (2024). Global Hydrogen Review 2024. https://www.iea.org/reports/global-hydrogen-review-2024 0
Li, J. (2022). Oxygen Evolution Reaction in Energy Conversion and Storage: Design Strategies Under and Beyond the Energy Scaling Relationship. Nano-Micro Letters, 14(1), 112. https://doi.org/10.1007/s40820-022-00857-x
Markandya, A., & Wilkinson, P. (2007). Electricity generation and health. Lancet, 370(9591), 979–990. https://doi.org/10.1016/s0140-6736(07)61253-7 Mathew, M. D. (2022). Nuclear energy: A pathway towards mitigation of global warming. Progress in Nuclear Energy, 143(104080), 104080. https://doi.org/10.1016/j.pnucene.2021.104080
National Grid. (2025). The hydrogen colour spectrum. Nationalgrid.com. https://www.nationalgrid.com/stories/energy-explained/hydrogen-colour-spectrum
Niu, S., Li, S., Du, Y., Han, X., & Xu, P. (2020). How to reliably report the overpotential of an electrocatalyst. ACS Energy Letters, 5(4), 1083–1087. https://doi.org/10.1021/acsenergylett.0c00321
Nørskov, J. K., Abild-Pedersen, F., Studt, F., & Bligaard, T. (2011). Density functional theory in surface chemistry and catalysis. Proceedings of the National Academy of Sciences of the United States of America, 108(3), 937–943. https://doi.org/10.1073/pnas.1006652108
Rashad, S. M., & Hammad, F. H. (2000). Nuclear power and the environment: comparative assessment of environmental and health impacts of electricity-generating systems. Applied Energy, 65(1–4), 211–229. https://doi.org/10.1016/s0306-2619(99)00069-0
Rosenow, J. (2022). Is heating homes with hydrogen all but a pipe dream? An evidence review. Joule, 6(10), 2225–2228. https://doi.org/10.1016/j.joule.2022.08.015
Song, K., & Le, D. (2013, October 2). Bond Energies. Chemistry LibreTexts; LibreTexts Chemistry. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_ Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/ Fundamentals_of_Chemical_Bonding/Bond_Energies
U.S. Department of Energy. (n.d.-a). DOE explains…Catalysts. U.S. Department of Energy. Retrieved January 25, 2025, from https://www.energy.gov/science/doe-explainscatalysts
U.S. Department of Energy. (n.d.-b). Hydrogen Storage. Energy.gov. Retrieved January 25, 2025, from https://www.energy.gov/eere/fuelcells/hydrogen-storage
U.S. Department of Energy. (2010). Hydrogen and Fuel Cell Technologies Program: Fuel Cells. https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/doe_h2_fuelcell_factsheet.pdf
U.S. Energy Information Administration. (2023, October 31). How electricity is generated. U.S. Energy Information Administration. https://www.eia.gov/energyexplained/electricity/how- electricity-is-generated.php
U.S. Energy Information Agency. (2024, June 21). Use of Hydrogen. U.S. Energy Information Agency. https://www.eia.gov/energyexplained/hydrogen/use-of-hydrogen.php
Wang, B., & Zhang, F. (2022). Main descriptors to correlate structures with the performances of electrocatalysts. Angewandte Chemie (International Ed. in English), 61(4), e202111026. https://doi.org/10.1002/anie.202111026
WHA International Inc. (2023, September 21). Top industrial uses of hydrogen, and the need for industrial hydrogen safety. WHA International, Inc. https://wha- international.com/hydrogen-in-industry/
World Nuclear Association. (2024, May 17). Hydrogen Production and Uses. World Nuclear Association. https://world-nuclear.org/information-library/energy-and-the- environment/hydrogen-production-and-uses
World Resources Institute. (2022). Climate Watch Historical Country Greenhouse Gas Emissions Data. Climate Watch. https://www.climatewatchdata.org/ghg-emissions
Yan, Z., Liu, H., Hao, Z., Yu, M., Chen, X., & Chen, J. (2020). Electrodeposition of (hydro)oxides for an oxygen evolution electrode. Chemical Science (Royal Society of Chemistry: 2010), 11(39), 10614–10625. https://doi.org/10.1039/d0sc01532f
Zheng, Y., Jiao, Y., Vasileff, A., & Qiao, S.-Z. (2018). The hydrogen evolution reaction in alkaline solution: From theory, single crystal models, to practical electrocatalysts. Angewandte Chemie (International Ed. in English), 57(26), 7568–7579.
About the author

Jiajun Li
Jiajun is currently a 12th grade student at St. Andrew’s College. He is interested in physics, specifically nuclear physics, as well as environmental science, specifically the energy aspect. Jiajun is currently investigating how a clean and renewable energy source can solve most of the environmental crises that we are currently facing and how to develop future energy sources, such as advanced fission reactors and nuclear fusion reactors, which could greatly benefit society.
Jiajun is the leader and founder of his school’s physics club and a vital member of the environmental council, which has made significant progress on helping the environment within his school, including reducing food waste by over 20%. At his previous school, three other students and Jiajun succeeded in installing a solar energy system, and he is also planning the installation of a larger solar power system to power his current school.
