
Author: Maggie McIntyre
Mentor: Dr. Hong Pan
Glenbard South High School
Abstract
Autoimmune diseases are chronic conditions in which the immune system mistakenly attacks healthy cells, tissues, and organs, leading to widespread inflammation and debilitating symptoms. Current treatments often rely on trial-and-error, leading to inconsistent outcomes and prolonged patient suffering. Emerging research highlights the role of the gut microbiome in regulating immune function, with diet serving as a key factor in shaping microbial composition and influencing immune responses. This connection has positioned nutrition as a promising strategy for restoring immune balance and improving autoimmune disease management.
Precision nutrition offers a more individualized approach compared to traditional dietary guidelines by considering unique patient characteristics such as genetics, gut microbiome composition, metabolism, and lifestyle. Whole diet strategies like the autoimmune protocol, Mediterranean diet, and plant-based diets, along with targeted nutrient interventions, demonstrate potential in reducing inflammation and enhancing treatment effectiveness. However, current research remains limited by small sample sizes, short study durations, and a lack of participant diversity, making it difficult to determine causation rather than correlation.
To address these limitations, multi-omics technologies and machine learning have emerged as powerful tools to advance precision nutrition. Multi-omics enables researchers to collect and integrate large-scale biological data, while machine learning identifies underlying patterns in the data and predicts patient responses to dietary interventions. Together, these methods support the development of data-driven, personalized nutrition strategies that move beyond one-size-fits-all care. By integrating diet, gut microbiome research, and computational modeling, precision nutrition has the potential to transform autoimmune disease management, improving both treatment outcomes and patient quality of life.
Key Terms
Autoimmune Disease – Conditions where the immune system is overactive, causing it to attack the body’s healthy cells, tissues, and organs. This leads to a variety of symptoms, such as fatigue, joint pain, and rashes.
Gut Microbiome – The collection of trillions of microorganisms and their genetic material living in the gastrointestinal tract (GIT). The gut microbiome plays a crucial role in digestion, immune function, and protecting the body from disease.
Precision Nutrition – Precision nutrition is an approach to developing comprehensive nutritional recommendations based on an individual’s characteristics, such as genetics, gut microbiome, physical activity, and dietary preferences, to improve individual health, prevent disease, and manage existing health issues.
Multi-Omics – The combination of different omics fields, such as genomics, metabolomics, and proteomics, is used to study biological processes and how they relate to health and disease. Researchers can use multi-omics methodologies to provide more personalized treatment options, like precision nutrition, to patients with autoimmune disease.
Machine Learning – A branch of Artificial Intelligence (AI) that uses computational methods to find patterns in data to make predictions and decisions. In microbiome research, machine learning canbe used to find links between diets, microbiota, and health outcomes.
Introduction
The immune system is a large network of cells, tissues, and organs that are responsible for defending the host against infections and diseases. In autoimmune diseases, the immune system becomes overactive and mistakenly attacks the body’s own healthy cells, tissues, or organs. These misdirected immune responses result in chronic inflammation, organ damage, and debilitating symptoms that significantly reduce quality of life.. The five most common autoimmune diseases worldwide include type 1 diabetes, rheumatoid arthritis (RA), psoriasis, multiple sclerosis, and inflammatory bowel disease (IBD). Current treatment approaches for autoimmune conditions often follow a trial-and-error process, potentially leading to ineffective treatments and additional patient suffering.
Diet, the gut microbiome, and the immune system are closely connected (Ross et al., 2024). Diet shapes the composition and functions of gut microbiota by determining which microbial species thrive and what products, or metabolites, they produce. In turn, the gut microbiome influences immune system activity through regulating immune cell function, producing SCFAs that regulate inflammation, and maintaining the intestinal barrier, which acts as a physical barrier for pathogens (Sasaki et al., 2024). A healthy gut microbiome is diverse and resilient to perturbations. Disruptions in the gut microbiome’s composition, a state known as dysbiosis, have been linked to the development and progression of autoimmune diseases. Given the impact that nutrition can have on the microbiome and immune system, precision nutrition has emerged as an approach to developing nutritional recommendations based on an individual’s characteristics, such as genetics, gut microbiome, physical activity, and dietary preferences, to manage autoimmune disease. However, many studies on dietary interventions or microbiome-targeted treatments are limited by small sample sizes, short time periods of study, and a lack of diversity in participant populations, making it difficult to establish causation rather than correlation.
Omics refers to a branch of research in biological sciences that studies communities of biological molecules and their functions within an organism. Omics methodologies allow researchers to analyze large sets of data from a biological system, such as genes (genomics), RNA transcriptions (transcriptomics), proteins (proteomics), and metabolites (metabolomics) (Whon et al., 2021). Multi-omics refers to the combination of multiple branches of omics to analyze the different complexities of a biological system. Multi-omics technologies and machine learning have the potential to address research limitations by making the collection and analysis of vast amounts of data much easier for researchers. Multi-omics methodologies allow researchers to gain a comprehensive understanding of biological communities and systems, like the gut microbiome. Machine learning can then be implemented to analyze the large data sets that multi-omics technologies produce, potentially finding patterns and making predictions that researchers would otherwise overlook. However, research that combines long-term microbiome tracking, detailed dietary assessments, and computational modeling to predict treatment outcomes is still required to successfully implement precision nutrition as a means of autoimmune management.
This paper addresses these gaps by exploring the role of diet in regulating immune responses for the treatment of autoimmune disease, and by discussing how multi-omics approaches coupled with machine learning can accelerate the development of personalized, data-driven dietary strategies for patients.
Autoimmune Disease and Gut Microbiome Interactions
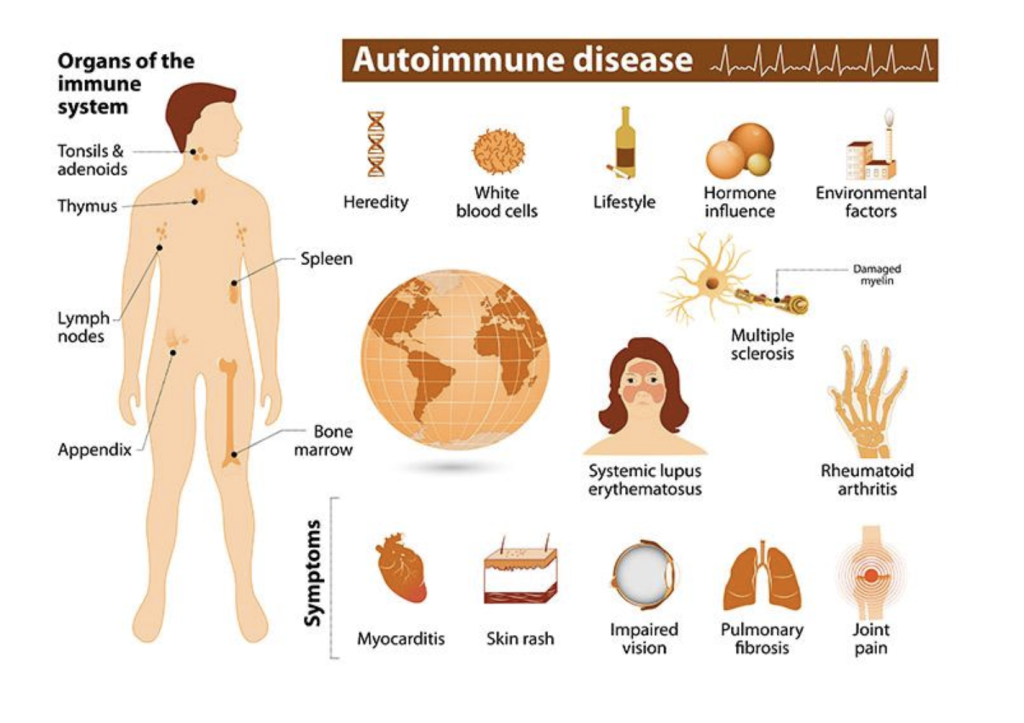
Autoimmune diseases are increasing in prevalence globally, especially in the United States, where 8% of the national population has been diagnosed with an autoimmune condition. Currently, therapy options for autoimmune patients proceed in a trial-and-error manner (Nayak & Orellana, 2024). This strategy can lead to ineffective therapies, resulting in suffering and further damage to patients. Multiple factors influence patients’ response to treatment, including genetics, age and gender, environmental factors (like diet and smoking), and concurrent therapies. Recent research has suggested that the gut microbiome is also a contributing factor in the development and progression of autoimmune disease (Ng & Lassere, 2025).
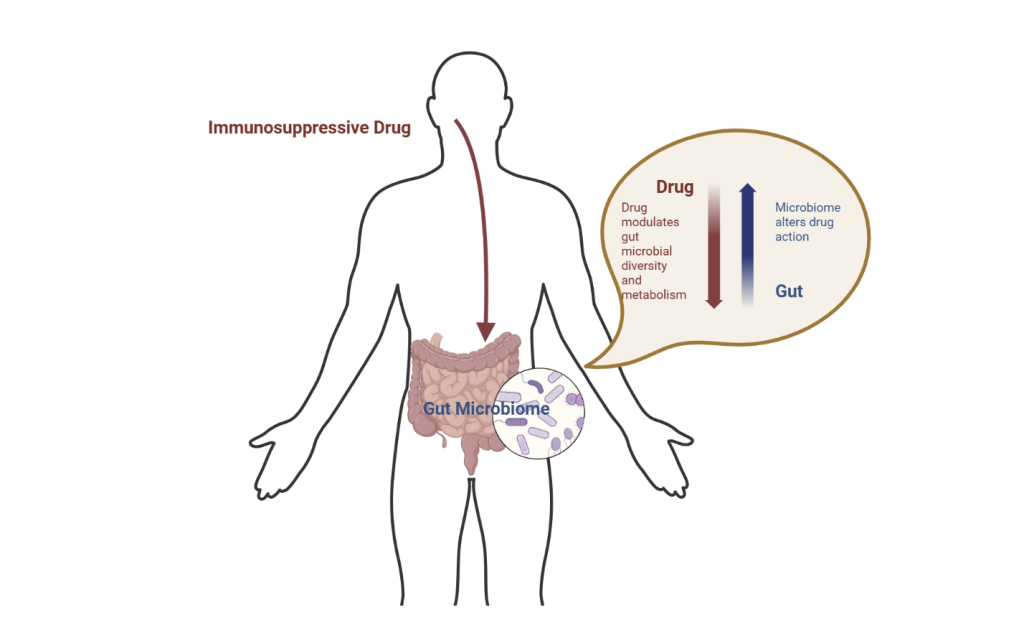
The gut microbiome and the immune system are constantly working together to maintain homeostasis. The gut microbiome influences the activity of various immune cells (T cells, B cells, and macrophages), produces metabolites (like SCFAs) that can have anti-inflammatory effects and influence immune cells’ function, and helps to maintain the health of the gut barrier, which is a crucial physical barrier against pathogens. Meanwhile, the immune system helps regulate the composition of the gut microbiome by preventing harmful bacteria from overgrowing and potentially triggering immune responses to microbial imbalances or dysbiosis (Ng & Lassere, 2025). Variations in gut microbial communities can influence host metabolism and immunity, contributing to differences in autoimmune treatment responses. One mechanism behind this variability is the activity of microbial enzymes, which are proteins produced by gut bacteria that can break down or modify compounds, including immunosuppressive medications. These enzymes can impact the effects of medication within the human body. Meanwhile, microbes influence a host’s response to immunosuppressive drugs through direct interaction with the drug, like microbial enzyme interactions, and indirectly by shaping the way the immune system responds to treatment. A case study on the drug Methotrexate (MTX), a significant piece of rheumatology due to its tolerability and cost-effectiveness, demonstrated that gut microbial composition plays a role in how patients metabolize and respond to MTX, potentially influencing both its effectiveness and side effects (Nayak & Orellana, 2024).
Research has shown that individuals with autoimmune disease often exhibit distinct microbial features when compared to those without autoimmune disease. In a meta-analysis of rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and reactive arthritis (ReA), all types of arthritis demonstrated distinct microbial features compared to healthy individuals, both in human and animal studies (Ng & Lassere, 2025). Reduced alpha diversity was present in several of the reviewed studies, demonstrating a lack of microbial diversity in arthritis patients. Additionally, several studies in the meta-analysis showed an abundance of Prevotella species, with lower levels of anti-inflammatory bacteria, like Akkermansia. Beta diversity was different from healthy controls, revealing microbial clustering in patients with arthritis. Dysbiosis was evident across all arthritis types, especially in RA and AS (Ng & Lassere, 2025).
Dietary Strategies to Restore Immune Homeostasis
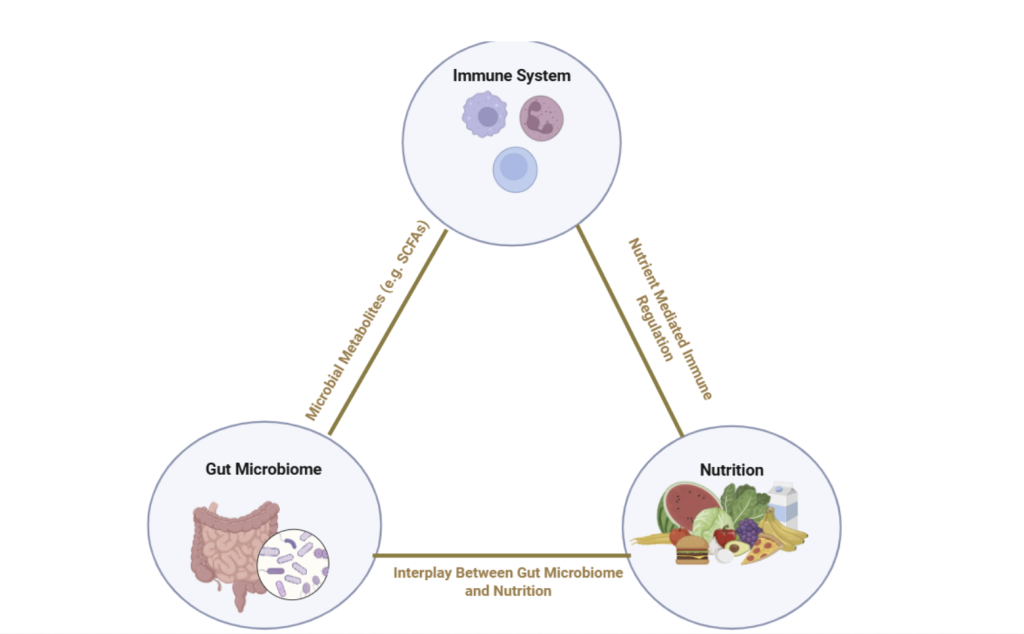
Diet is a key factor influencing the composition and function of the gut microbiota (Ross et al., 2024). Because the gut microbiome plays a central role in regulating immune function, diet has emerged as a strategy for restoring immune homeostasis in patients with autoimmune diseases. What a person eats not only affects the availability of nutrients for the body but also determines which microbial species thrive in the gut and what metabolites they produce. These microbial byproducts, such as SCFAs, play key roles in regulating immune cell activity, reinforcing the gut barrier, and reducing chronic inflammation. Diets tailored to reduce inflammation and address nutrient deficiencies can significantly help manage autoimmune disease symptoms. Not only can diet directly regulate patients’ immune activity, but it can also change the way that the patient responds to immunosuppressive drug treatment.
In terms of using diet as a tool to manage autoimmune disease, there are two primary approaches. The first involves a whole diet approach, which emphasizes broad dietary patterns that encourage microbial diversity and reduced inflammation (Bagur et al., 2017). Dietary patterns used by autoimmune patients emphasize nutrient-dense, whole foods while eliminating processed foods, added sugars, and potential food sensitivities. Three primary diets that meet this criterion include the Autoimmune Protocol (AIP) diet, the Mediterranean diet, and a plant-based diet. The AIP diet is an elimination-based diet designed to reduce inflammation by having patients exclude foods that might trigger immune responses. The Mediterranean diet (MD) emphasizes whole, unprocessed foods, specifically plants, healthy fats, and moderate consumption of fish, poultry, and dairy, with a low intake of red meat and refined sugar (Ross et al., 2024). The MD is rich in antioxidants and fiber, which can help modulate the immune system and reduce inflammation. The plant-based diet takes an elimination approach to the AIP diet, eliminating animal products, which can be common dietary triggers. Like the MD, it is high in fiber, antioxidants, and phytochemicals, offering comparable anti-inflammatory benefits.
In addition to whole dietary patterns, targeted nutrient-level interventions can play a critical role in managing autoimmune disease. Specific nutrients have been shown to influence immune cell function, reduce inflammation, and support a healthy gut microbiome. Several vitamins can be beneficial for individuals with autoimmune disease, including vitamin D, vitamin B6, vitamin B12, and omega-3 fatty acids. For example, vitamin D plays a significant role in regulating immune function, immune cell activation, and the production of anti-inflammatory cytokines (Wimalawansa, 2023). Deficiency in vitamin D is common among individuals with autoimmune conditions, and supplementation has been associated with reduced disease activity in some studies (Wimalawansa, 2023). In addition to vitamin D, vitamins B6 and B12 are extremely important in immune system health and inflammation management. Vitamin B6 is involved in immune cell production, antibody production, and white blood cell regulation. Vitamin B12 is necessary for the production of white and red blood cells, but it also supports neurological health and the regulation of homocysteine, a compound that, when elevated, is associated with autoimmune disease (Bagur et al., 2017). Ensuring adequate intake of vitamin B6 and B12 may support immune function and reduce disease flare-ups in autoimmune patients. Finally, Omega-3 fatty acids exhibit similar anti-inflammatory properties and show potential benefits for autoimmune diseases such as psoriasis, multiple sclerosis, juvenile arthritis, IgA nephropathy, Crohn’s disease, and ulcerative colitis (Bagur et al., 2017).
Precision Nutrition through Multi-Omics and Machine Learning
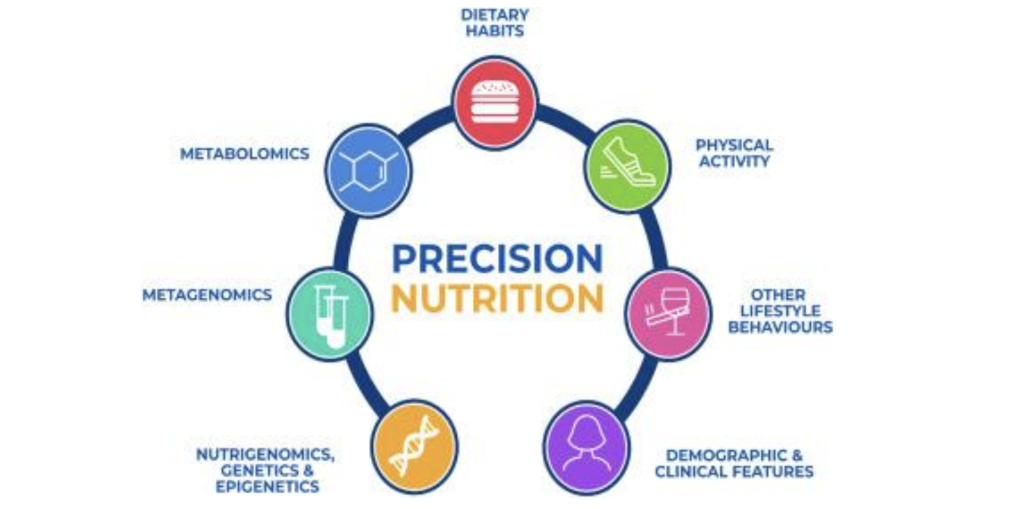
Fig 4. Figure sourced from Livingstone et al., 2022. Precision nutrition integrates multi-omics data, including genomics, proteomics, metabolomics, and microbiome profiling, to generate individualized dietary recommendations. This approach tailors interventions to each patient’s unique biology, lifestyle, and gut microbial composition, aiming to improve treatment outcomes and quality of life in autoimmune disease management. Machine learning enables this process by identifying patterns in large datasets and predicting patient responses to specific dietary strategies
Precision nutrition (PN), also known as personalized nutrition, is an approach to developing comprehensive nutritional recommendations based on an individual’s characteristics, such as genetics, gut microbiome, physical activity, and dietary preferences (Kirk et al., 2021). The primary aim of PN interventions is to improve individual health, prevent disease, and manage existing health issues. Unlike traditional dietary guidelines that follow a one-size-fits-all approach, PN considers the fact that different individuals may respond differently to the same dietary patterns. In autoimmune management, PN can offer a more specific and personalized approach to dietary interventions, which is crucial given that current therapies often proceed in a trial-and-error manner, leading to ineffective treatments and patient suffering.
To achieve the level of treatment personalization that PN requires, researchers and medical practitioners rely on multi-omics profiling to gather large amounts of biological data (Kirk et al., 2021). Multi-omics combines data from various omics fields, like genomics, proteomics, and metabolomics, to gain a detailed, big-picture view of the biological processes underlying autoimmune disease. To illustrate, using multi-omics data, researchers can analyze a patient’s gut microbiome, along with their genes, protein production, and metabolite production, to better understand how specific diets or nutrients might impact their immune responses. The immense amount of data generated by multi-omics technologies requires computational analysis methods (Kruta et al., 2024).
This is where machine learning (ML) becomes essential. ML is a branch of artificial intelligence that uses computational methods to find patterns in data to make predictions and decisions (Kirk et al., 2021). In developing treatments for autoimmune disease, ML algorithms can be implemented to identify underlying patterns in multi-omics data to make diagnoses and predict how patients with autoimmune disease respond to dietary or drug interventions. For example, Kruta et al. developed a machine learning framework that achieved a high prediction accuracy of up to 96% in classifying and diagnosing autoimmune diseases. The study also found that metabolomic data was the most important factor in predicting the diagnosis of Rheumatoid Arthritis (RA).
Discussion
The relationship between autoimmune diseases and the gut microbiome has become more prevalent in recent research, bringing about both promises and limitations. The emerging research regarding the gut microbiome’s role in autoimmune disease creates many opportunities for early detection and prevention. Reduced microbial diversity and higher dysbiosis scores have been linked to greater disease severity in many studies, as seen in conditions such as RA, PsA, and pSS (Ng & Lassere, 2025). This supports the idea that whole diet approaches or nutrient supplementation could help restore immune balance. However, the main limitations of current research exploring the microbiome in autoimmune disease are the small sample size of most studies, the lack of comparison to healthy controls in certain groups, and the lack of longitudinal data, making it challenging to determine causation rather than correlation.
Additionally, further implementation of multi-omics methodologies in gut microbiome studies offers a promising route for biomarker discovery in autoimmune disease. For instance, one study identified butyric acid, propionic acid, and acetic acid as metabolites involved in rheumatoid arthritis-related immune pathways, while another detected 20 metabolites in plasma or urine that could be used in predictive models for AS (Wang et al., 2016). In autoimmune thyroid disease (AITD), metabolism changes may alter microbiota composition, though findings so far show association without confirmed causation (Gong et al., 2021).
Dietary interventions, such as the Autoimmune Protocol (AIP), Mediterranean diet, and plant-based diets, can potentially reduce inflammation, improve gut health, and enhance drug response for patients with autoimmune disease. Beyond autoimmune disease, AIP has been applied to conditions like eczema, eosinophilic esophagitis, and even ADHD, demonstrating the diet’s broader potential. Precision nutrition offers an even more personalized approach to diet-based treatment methods. By utilizing multi-omics technologies, vast amounts of gut microbiome data can be analyzed to understand its relationship with the immune system (Chen et al., 2024). Machine learning models can analyze these multi-omics datasets to identify diagnostic biomarkers, predict treatment responses, and personalize dietary recommendations (Chen et al., 2024). Future research should prioritize larger, longitudinal studies that combine these approaches to move autoimmune care from trial-and-error toward individualized, data-driven approaches.
Conclusion
Autoimmune diseases result from a combination of genetic, environmental, and lifestyle factors, with the gut microbiome playing a primary role in modulating immune responses. Diet directly influences microbial composition and metabolite production, which in turn regulate inflammation and treatment outcomes. Dietary strategies such as the Autoimmune Protocol, Mediterranean, and plant-based diets show potential in reducing inflammation and restoring balance, yet current research remains limited by small sample sizes, short study periods, and a lack of participant diversity.
To overcome these limitations, precision nutrition approaches supported by multi-omics technologies and machine learning are emerging in the field of microbiome research. Multi-omics offers a comprehensive understanding of biological systems, while machine learning aids in the identification of patterns in omics data and the prediction of autoimmune treatment responses. By utilizing these approaches, future research can move autoimmune care away from trial-and-error toward personalized strategies that improve outcomes and quality of life for patients.
Acknowledgements
I would like to thank Dr. Hong Pan at Simmons University for his mentorship throughout my research process.
References
Bagur, M. J., Murcia, M. A., Jiménez-Monreal, A. M., Tur, J. A., Bibiloni, M. M., Alonso, G. L., & Martínez-Tomé, M. (2017). Influence of Diet in Multiple Sclerosis: A Systematic Review. Advances in Nutrition, 8(3), 463–472. https://doi.org/10.3945/an.116.014191
Carpi, R. Z., Barbalho, S. M., Sloan, K. P., Laurindo, L. F., Gonzaga, H. F., Grippa, P. C., Zutin, T. L. M., Girio, R. J. S., Repetti, C. S. F., Detregiachi, C. R. P., Bueno, P. C. S., Mazuqueli Pereira, E. de S. B., Goulart, R. de A., & Haber, J. F. dos S. (2022). The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. International Journal of Molecular Sciences, 23(15), 8805. https://doi.org/10.3390/ijms23158805
Chen, L., Zhang, C., Niu, R., Xiong, S., He, J., Wang, Y ., Zhang, P., Su, F., Liu, Z., Zhou, L., Mao, R., Hu, S., Chen, M., Qiu, Y ., & Feng, R. (2024). Multi‐Omics Biomarkers for Predicting Efficacy of Biologic and Small‐Molecule Therapies in Adults With Inflammatory Bowel Disease: A Systematic Review. United European Gastroenterology Journal, 13(4), 517–530. https://doi.org/10.1002/ueg2.12720
Gong, B., Wang, C., Meng, F., Wang, H., Song, B., Yang, Y ., & Shan, Z. (2021). Association Between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis. Frontiers in Endocrinology, 12. https://doi.org/10.3389/fendo.2021.774362
Kirk, D., Catal, C., & Tekinerdogan, B. (2021). Precision nutrition: A systematic literature review. Computers in Biology and Medicine, 133, 104365. https://doi.org/10.1016/j.compbiomed.2021.104365
Kruta, J., Carapito, R., Trendelenburg, M., Martin, T., Rizzi, M., V oll, R. E., Cavalli, A., Natali, E., Meier, P., Stawiski, M., Mosbacher, J., Mollet, A., Santoro, A., Capri, M., Giampieri, E., Schkommodau, E., & Miho, E. (2024). Machine learning for precision diagnostics of autoimmunity. Scientific Reports, 14(1), 27848. https://doi.org/10.1038/s41598-024-76093-7
Livingstone, K. M., Ramos-Lopez, O., Pérusse, L., Kato, H., Ordovas, J. M., & Martínez, J. A. (2022). Precision nutrition: A review of current approaches and future endeavors. Trends in Food Science & Technology, 128, 253–264. https://doi.org/10.1016/j.tifs.2022.08.017
Nayak, R. R., & Orellana, D. A. (2024). The impact of the human gut microbiome on the treatment of autoimmune disease. Immunological Reviews, 325(1), 107–130. https://doi.org/10.1111/imr.13358
Ng, B. C. K., & Lassere, M. (2025). The role of the gastrointestinal microbiome on rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and reactive arthritis: A systematic review. Seminars in Arthritis and Rheumatism, 70, 152574. https://doi.org/10.1016/j.semarthrit.2024.152574
Ross, F. C., Patangia, D., Grimaud, G., Lavelle, A., Dempsey, E. M., Ross, R. P., & Stanton, C. (2024). The interplay between diet and the gut microbiome: implications for health and disease. Nature Reviews. Microbiology, 22(11), 671–686. https://doi.org/10.1038/s41579-024-01068-4
Sasaki, M., Suaini, N. H. A., Afghani, J., Heye, K. N., O’Mahony, L., Venter, C., Lauener, R., Frei, R., & Roduit, C. (2024). Systematic review of the association between short-chain fatty acids and allergic diseases. Allergy. Advance online publication. https://doi.org/10.1111/all.16065
Wang, W., Yang, G., Zhang, J., Chen, C., Jia, Z., Li, J., & Xu, W. (2016). Plasma, urine and ligament tissue metabolite profiling reveals potential biomarkers of ankylosing spondylitis using NMR-based metabolic profiles. Arthritis Research & Therapy, 18(1), 244. https://doi.org/10.1186/s13075-016-1139-2
Wang, X., Liu, X., Gong, F., Jiang, Y ., Zhang, C., Zhou, W., & Zhang, W. (2025). Targeting gut microbiota for diabetic nephropathy treatment: probiotics, dietary interventions, and fecal microbiota transplantation. Frontiers in Endocrinology, 16, 1621968. https://doi.org/10.3389/fendo.2025.1621968
Wimalawansa, S. J. (2023). Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review. Nutrients, 15(17), 3842. https://doi.org/10.3390/nu15173842
Whon, T. W., Shin, N.-R., Kim, J. Y ., & Roh, S. W. (2021). Omics in gut microbiome analysis. Journal of Microbiology (Seoul, Korea), 59(3), 292–297. https://doi.org/10.1007/s12275-021-1004-0
About the author

Maggie McIntyre
Maggie is a junior at Glenbard South High School with a passion for math and computer science. She teaches coding and robotics classes to elementary and middle school students, competes on her school’s math team, and enjoys baking and cooking in her free time.
