
Author: Maggie Zaidman
Franklin Delano Roosevelt
Abstract
This study examined the effect of glucose concentration on carbon dioxide (CO₂) production during yeast fermentation to determine the optimal sugar level for maximizing anaerobic respiration. Six trials were conducted using 500 mL bottles containing 200 mL of water at 30°C, 7 g of dried yeast, and varying glucose amounts (0–25 g). CO₂ output was indirectly measured by recording the circumference of balloons attached to each bottle at 10-minute intervals over 50 minutes. Results showed that moderate glucose concentrations (10–15 g) produced the greatest CO₂ output, with the 10 g trial yielding the highest balloon circumference increase (21.4 cm). Lower (0–5 g) and higher (20–25 g) glucose concentrations resulted in reduced CO₂ production, potentially due to substrate limitation or ethanol inhibition. The findings suggest a threshold beyond which additional glucose may hinder fermentation efficiency. Despite limitations—including a lack of repeated trials and balloon leakage—this experiment highlights the importance of glucose optimization in fermentation-based industries.
Purpose
How does the amount of sugar affect the amount of CO₂ released in a bottle with water, sugar, and yeast in a span of 50 minutes?
Background information
In order for cells to receive the necessary quantity of energy for functioning, in the absence of oxygen (O₂), there must be an anaerobic respiration process classified as “fermentation”, in which sugars, such as glucose and fructose (C₆H₁₂O₆) produce changes in organic substrates in order to provide energy (ATP) for cells. “Glycolysis is a cytoplasmic pathway which breaks down glucose into two three-carbon compounds and generates energy” (Kumari, 2018). This indicates that glycolysis is the initial stage of converting glucose into energy for cellular metabolism. This must occur prior to fermentation, since without the breakage of sugars by cells into two smaller sugar molecules( of a chemical called pyruvate) in enzyme reactions, ATP wouldn’t be produced since the splitting of the cells is what initially produces energy. Fermentation has been a crucial part of science for human evolution since it has allowed the formation of edible elements such as yogurt, wine, beer, miso, kimchi, and bread. All of these processes have been performed because of the usage of yeast (C19H14O2 – bakers yeast [most common type of yeast]) combined with sugars, which later create ethanol (C₂H₆O – also known as alcohol) and carbon dioxide (CO₂). “An important feature of yeasts (especially Saccharomyces cerevisiae – a.k.a. S. cerevisiae) is that their cells […] are eukaryotic – meaning they have a nucleus containing DNA packaged into chromosomes. And although it may seem like yeast and humans have very little in common, at least 20 percent of human genes known to have a role in disease have counterparts in yeast.” (FenoLogica, 2017). This indicates that the study of yeast allows the improvements of medical analysis and advances because of the similarity within yeast and human cells. The investigation of yeast and fermentation is substantial since finding a more rapid fermentation procedure can increase the production ofthe yeast-based products mentioned previously, which could positively impact the global economy, raising the markets of yeast-made products.
This specific investigation focuses on how the quantity of sugar (C₆H₁₂O₆) additioned to a mixture of yeast and water on a 500mL bottle impacts the CO₂ production of fermentation. The independent variable chosen was the sugar concentration in the experiment. This is because sugars are the main stimuli for fermentation to occur, and incrementing/decreasing the sugar concentration added per experiment trial may impact the speed of production of CO₂molecules. The dependent variable in this experiment is the rate of CO₂ production and/or the circumference of the balloon. This is because the production of CO₂ will be dependent on the amount of sugar (in this experiment) added to the yeast/water mixture since the chemical element that is released after fermentation is CO₂ . The quantities of sugar added per trial (bottle) were calculated in a 5-by-5 matter, beginning at 5g (including control variable: 0g), and ending at 25g, having 6 trials overall (including control). This was chosen as an effective measure of sugar quantities so that there wouldn’t be too little nor too much sugar in each trial. “In the 1850s and 1860s, the French chemist and microbiologist Louis Pasteur became the first scientist to study fermentation, when he demonstrated that this process was performed by living cells. Fermentation processes to produce wines, beers and ciders are traditionally carried out with Saccharomyces cerevisiae strains, the most common and commercially available yeast.” (Maicas, 2020). According to the previous passage, the study of fermentation had been intentional for the past 173-163 years, and indicates that fermentation can be produced with traditionally-known yeast. This means that we should continue to investigate it since “genetic manipulation in yeast is easy and cheap compared to similar experiments in more complex animals such as mice and zebrafish” (YourGenome, 2021). With yeast, one can do complex experiments regarding various topics in an easy, efficient way.
C6H12O6 (aq) ————> 2C2H5OH (aq) + 2CO2(g) + 2ATP
Hypothesis
If glucose levels impact the quantity of CO₂ production during fermentation, the more glucose (sugars) added to a 30* celsius water-yeast experiment, the higher the anaerobic respiration rate, the more CO₂ produced.
Variables
Independent variable (Manipulated variable):
Concentration of Sugar
Dependent variable (Measured variable):
Balloon Size (rate of CO₂ production)
Control variables:
Amount of water (200mL), amount of yeast (7g), size of bottles, temperature of water, temperature of the environment tested in, time of the day tested in , size + type of the balloons, same measuring tools per experiment.
Materials
- Dried yeast (total of 42 grams)
- 6 empty 500mL water bottles
- Granulated sugar (total of 75 grams)
- 6 Same size balloons
- Graduated cylinder
- Water
- Funnel
- String
- Ruler
- Tape
- Marker Pen
- Timer
- Thermometer
- Weightboat
- Mass
- A notepad and pen
Procedure
- 1. Place all materials listed on a stable surface
- 2. Place short strips of tape on each bottle
- 3. With a marker, label bottles as corresponding Bottle A = 5g, Bottle B = 10g, Bottle C = 15g, Bottle D = 20g, Bottle E = 25g and Control = 0g
- 4. Take the temperature of the water available for testing.
- 5. Measure 200mL of water using the graduated cylinder
- 6. Pour the water onto a bottle using a funnel
- 7. Repeat steps 4-5 until all bottles contain 200mL of water
- 8. Weight 5g of sugar with the weightboat
- 9. Add the 5g of sugar to Bottle A
- 10. Weight 10g of sugar using the weightboat
- 11. Add the 10g of sugar to Bottle B
- 12. Weight 15g of sugar using the weightboat
- 13. Add the 15g of sugar to Bottle C
- 14. Weight 20g of sugar using the weightboat
- 15. Add the 20g of sugar to Bottle D
- 16. Weight 25g of sugar using the weightboat
- 17. Add the 25g of sugar to Bottle E
- 18. Weight 7 grams of dried yeast on the mass/scale with the weightboat
- 19. Add the 7 grams of yeast to a bottle using the funnel
- 20. Repeat steps 18-19 until all bottles have 7g of yeast each
- 21. Attach one balloon on the open top of each bottle to close it
- 22. Gently raise each bottle and shake it for at least 15 seconds – place bottles down after shaking is done
- 23. Annotate observations plus circumference (size) of all balloons before initiating trials in a notepad24. Start a timer for 50 minutes utilizing a cell phone/computer
- 25. After every 10 minutes (until 50 minutes), use a string and a ruler to measure the circumference of
- each water bottle-balloon attachment and annotate in a notepad (place string around balloon and
- measure string’s length with the ruler)
- 26. Write down observations of every water bottle-balloon attachment on the notepad
- 27. End the experiment after 50 minutes
- 28. Rewrite the data + observations placed in the notepad onto the data table
- 29. When finishing experiment, discard ingredients and clean up the lab area
Data and Observations
Circumference of Balloons Depending on Sugar Amount
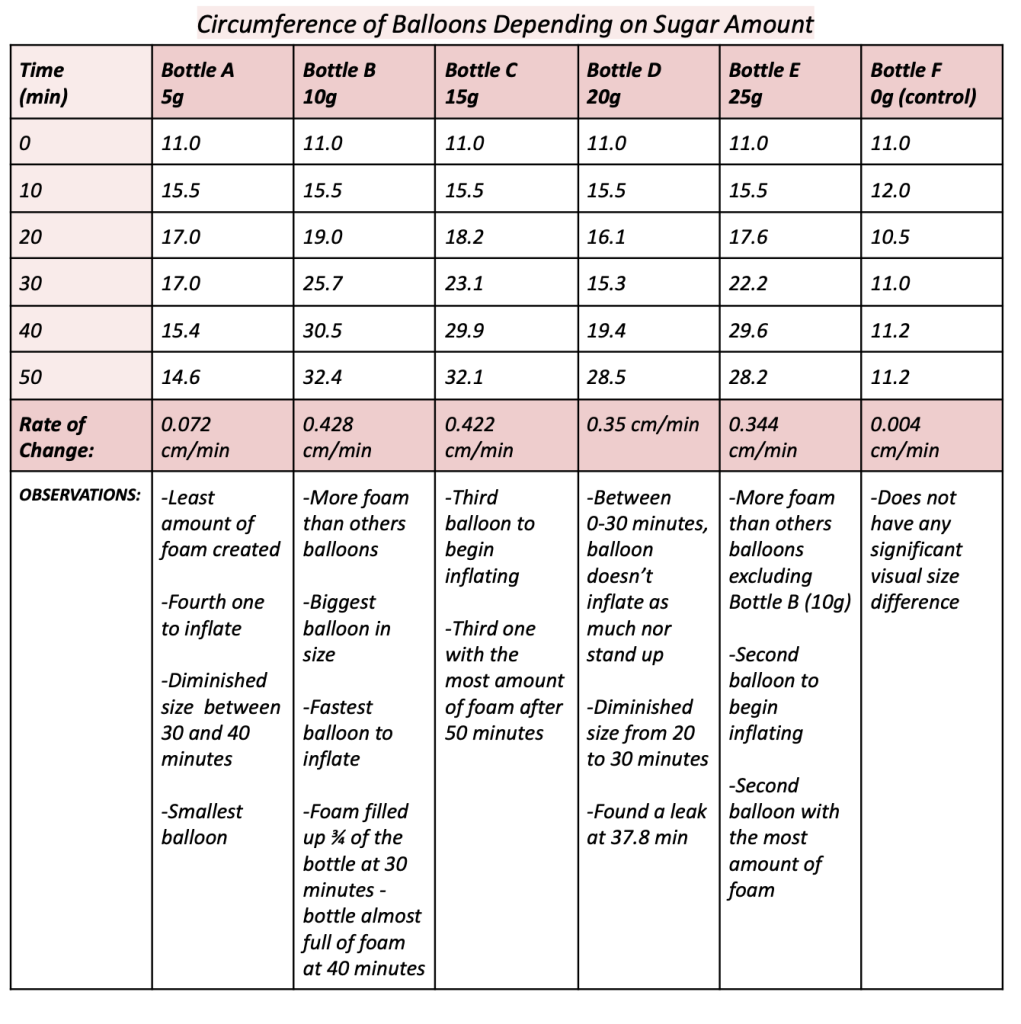
All the bottle’s liquid turned foggy and foam was created.



Conclusion
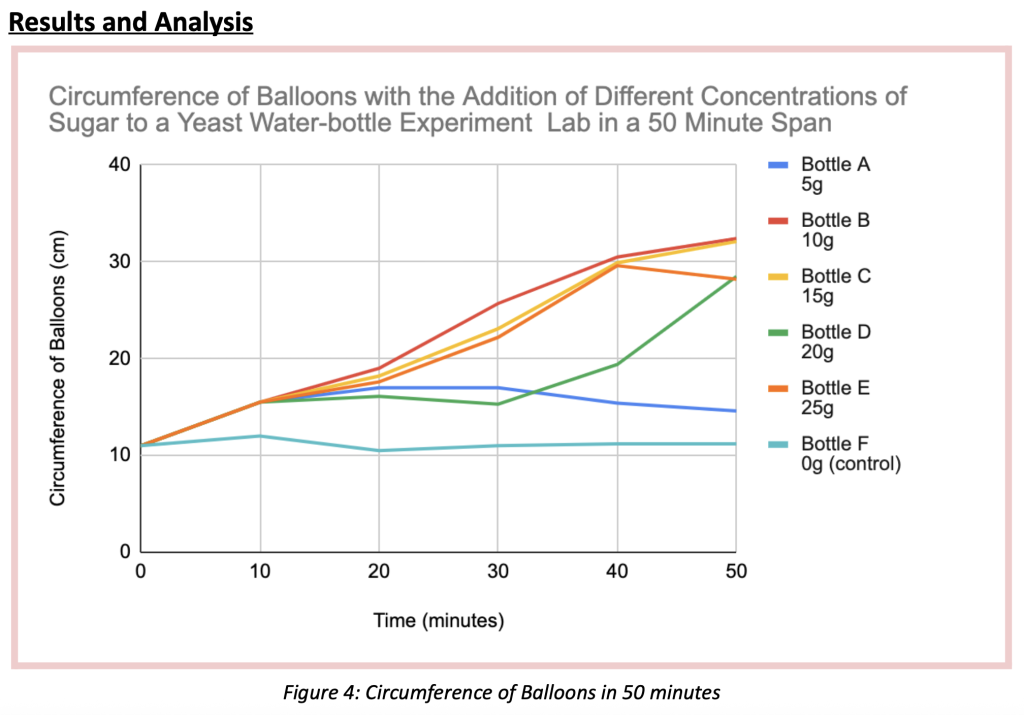
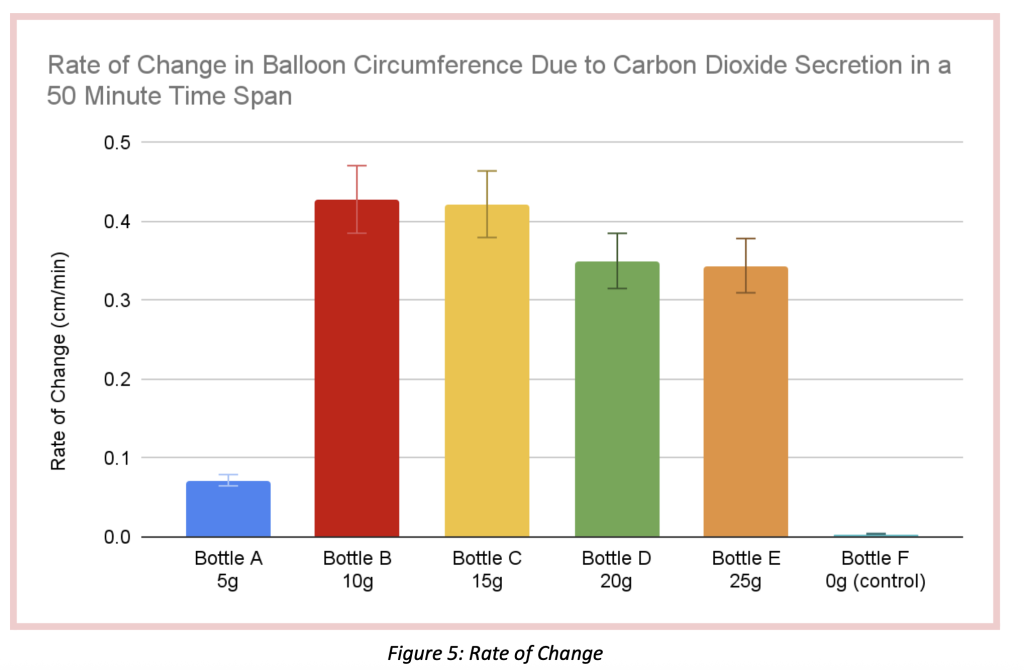
The purpose of this experiment was to determine if the concentration of sugar added to a 200mL 30*celsius water and 7g yeast solution (inside a bottle) impacted the rate of CO₂ production in fermentation during a span of 50 minutes. There was one trial held for this experiment, with 5 testing elements. In the 5 testings done with 5 different 750ml bottles, all containing the water-yeast mixture, Bottle A, contained 5g of sugar, Bottle B, 10g, Bottle C, 15g, Bottle D, 20g, and finally Bottle E contained 25g of sugar. To measure the amount of CO₂ produced per bottle, a balloon was placed and secured on the opening of every bottle, and the circumference was measured every 10 minutes (until reaching 50) with the utilization of a string and a ruler. For all bottle trials, the initial balloon circumference was 11cm. The balloon circumference that after 50 minutes grew the most was Bottle B, containing 10 grams of sugar. This balloon grew until it reached a 32.4 cm circumference, growing a total of 21.4 centimeters. On the other hand, the balloon with the least growth was Bottle E, containing no sugar at all. This balloon grew 0.2 cm, reaching a circumference of 11.2cm total. The order of balloons with the biggest circumference according to the data table and figure 4, from least to greatest were: 0g of sugar (11.2 circumference – 0.2cm growth – 0.004 cm/min rate of change), 5g of sugar (14.6 circumference – 3.6cm growth- 0.072 cm/min rate of change), 25g of sugar (28.2cm circumference – 17.2cm growth – 0.344 cm/min rate of change), 20g of sugar (28.5cm circumference, 17.5cm growth- 0.35 cm/min rate of change), 15g of sugar (32.1cm circumference – 21.1cm growth – 0.422 cm/min rate of change) and 10g of sugar (32.4 circumference – 21.4cm growth- 0.428 cm/min rate of change). This study rejects the original hypothesis, which claimed that as sugar was added, CO₂ production increased, increasing the balloon’s circumference. As shown in the data above, bottles with little sugar (0–5g) had a slower rate of CO₂ production. Unexpectedly, the bottles with high sugar content (20–25g) had a higher rate of CO₂ production than those with low sugar content, but they didn’t reach the same levels of CO₂ production as the bottles with 10-15g of sugar. This is because “ too much sugar can result in yeast that becomes stressed out and gets overwhelmed by ethanol production, potentially creating too much alcohol, which will kill off the yeast, also halting the fermentation process.”(Oculyze, 2022). This suggests that the first step in fermentation, glycolysis, cannot take place as intended because there are too many sugar molecules (C6H12O6) breaking down and producing large amounts of ethanol (C2H6O), which interfere with the ability of the yeast to break down sugar molecules and produce carbon dioxide (O₂) since ethanol becomes the dominant element in the mixture. Although any amount of sugars (glucose, fructose) will cause fermentation to occur, a moderate amount of sugar, about a ratio of 10-15 grams per every 200 ml of water and 7g of yeast, will produce more carbon dioxide according to the experiment. With this process, C₆H₁₂O₆ and C19H14O2 were transformed into C₂H₆O and CO₂.
Evaluation
In this investigation, there was a not-sufficient data collection, leading to an inconclusive claim. There should have been at least 4-5 trials in order to obtain a solid conclusion, yet there was only one with multiple inconveniences and errors. As the sugar was being massed (to reach 10 grams), water fell into the sugar which is believed to be a possible cause of the high and accelerated CO₂ production since a sugar-water mixture was activated prior to being added to the yeast-water solution. Additionally, two leaks were found. The first leak was found at 37.8 minutes in the bottle containing 20 grams of sugar, which was later patched with tape. The second one was found at 50 minutes in the bottle with 25 grams of sugar, during the laboratory clean up. The conclusion stated in this experiment was that there has to be a moderate ratio of sugar (in the experiment’s specific case, 10-15g) in order for the sugar to activate yeast to the maximum in order for more CO₂ to be produced since when too little sugar was added to the solution, there was a small growth, while when there was a lot of sugar added, the balloons grew, yet not as much as those with considerably moderate amounts of sugar. This scientific mechanism, called saturation, usually occurs with larger amounts of sugar in bigger experiments, so it happening with the 200ml water and 7g yeast mixture is unusual. Thus, the results being this way could be because of the leaks found on the balloons, since the 20g of sugar balloon, after being patched, grew rapidly. The leakage led to many variations in the data collected, which influenced the conclusion and final results. Many of the variables tested were not controlled such as the temperature of the environment, elements such as air conditioning being on, and the surface tested at. These variables were environmental, meaning lab workers had no control over them.
To have more accurate results, the data of CO₂ production should have been collected using a CO₂ probe, and the experiment should have been done in a more careful way, checking for possible leaks every 5-10 minutes.
Discussion
This investigation and any yeast investigation is crucial for the global economy since yeast, as mentioned while introducing the experiment, is necessary for the production of beverages and edibles like bread, miso, kimchi, and beer which are common and very sold in supermarkets. With this investigation, the producers of these elements could benefit from knowing when the sugars activate the yeast in these elements, since it would cause a more rapid production of them. One way to make the results in this experiment more reliable, guaranteeing a more solid base of information for yeast-involved edible producers would be to increment the quantities of sugar and testing with more bottles (20 approximately) which would allow producers to know when saturation occurs in order to prevent adding too much sugar to the ratio of other elements such as yeast and water previously established. A second investigation that could be beneficial would be having the same experiment with larger quantities and concentrations of water, sugar, and yeast with larger containers for testing (plus more trials). Large industries usually utilize large amounts of materials for production, so testing with more quantities would provide a better and more representative insight on how the quantities of sugar impact CO₂ production from a yeast-activated solution in a factory-environment.
References
Glycolysis – an overview | ScienceDirect Topics. (n.d.). Retrieved March 12, 2023, from www.sciencedirect.com/topics/neuroscience/glycolysis
Maicas, S. (2020). The Role of Yeasts in Fermentation Processes. In PubMed Central (PMC). Multidisciplinary Digital Publishing Institute (MDPI). www.ncbi.nlm.nih.gov/pmc/articles/PMC7466055/
Tarziu, C. (n.d.). How Does Ethanol Affect Yeast Fermentation? In Oculyze. Retrieved March 22, 2023, from www.oculyze.net/how-does-ethanol-affect-yeast-fermentation/
Why use yeast in research? (n.d.). In @yourgenome · Science website. Retrieved March 13, 2023, from www.yourgenome.org/facts/why-use-yeast-in-research/
Why Yeast is Important to Scientific Discovery — . (2017, August 29). www.fenologica.com/
.
About the author

Maggie Zaidman
Maggie is a dedicated student with a strong interest in neuroscience, biology, and medicine. Maggie’s research spans Alzheimer’s disease, synthetic biology, and aplastic anemia, and she is a 2024 Yale Young Global Scholars alumna.
As the founder of the organization EmpoderadaMente, Maggie empowers women in Perú by making neuroscience accessible. Through her initiative, she authored Conoce tu Mente, Mejora tu Vida, a book that explains neuroplasticity and brain health in practical terms. Maggie also collaborated in the iGEM FDR-HB team, where she played a key role in a synthetic biology project that engineered E. coli to degrade PET plastic and convert it into ethanol, aiming to create a sustainable energy solution for low-income communities.
Maggie aspires to bridge the gap between scientific research and patient-centered care, using her skills to drive positive change in both healthcare and community outreach.
