
Author: Rishya Gutti
Mentor: Fabiola Munarin
Neuqua Valley High School
Intro
Over the course of many years, stem cell therapies have been developed for regenerative medicine, but one of the main challenges remains how they should be delivered. When delivering any treatment method, getting as close to the affected area and using as targeted of a therapy as possible is one of the best ways to garner results. When targeted therapies are used, the drug or therapy becomes increasingly accessible to the tissues that are targeted (Levin, et.al), making the treatment that much more effective since the cells are able to gain the most benefit.
Targeted stem cell delivery offers unique advantages related to the particular nature of stem cells, which function as blank slates, meaning they can take on the form and function as the type of cell they are needed to be (Zakrzewski, et.al). Furthermore, they are able to differentiate into a multitude of cell types, depending on the regenerative signals that are expressed by the surrounding cells or tissues. Stem cell therapies have long been looked into in terms of various tissues, and some examples of these are ocular, cardiovascular, and osseous tissues, to facilitate regrowth or regeneration (Zakrzewski, et.al).
Some of the advantages of the injection procedures compared to open-wound surgery include that these are minimally invasive which leads to a less traumatic treatment which also minimizes the post-procedure complications for the recipient due to there not being large open wounds.
In terms of delivery methods, stem cells have been injected in vehicle media, hydrogels, and in microspheres/microtissues. Injection of stem cells in a vehicle medium is the most direct method, but cells may sustain damage. However, by immobilizing them into a biomaterial, such as a hydrogel or microsphere/microtissue, the stem cells are protected in a more complex environment during the implantation process. (Ashammakhi, et.al)
Injectable Stem Cells
The basis of implantation of stem cells in a minimally invasive manner is injection. This is one of the best ways to reduce the invasiveness to deliver the cells to their target. Stem cells suspensions can be delivered directly, through a syringe and needle. This, however, can pose a threat to the cells themselves as there is nothing but a saline solution protecting them from the pressure induced by the needle during the implantation process and causing damage to the cell walls. If this happens, a portion of the cells delivered may not be viable and therefore decrease the efficacy of the treatment (Ashammakhi, et.al). Notwithstanding, the size of the needle can be altered to prevent this occurrence. An optimal size needle (16-22 gauge) is used so that the stress on the cells is minimized. Large needles, on the other hand, could cause sediment and therefore affect the viability and homogeneity of dispersion of the cells as well (Ashammakhi, et.al).
One application of direct injection is in osteoarthritic patients, who need the treatment to help their deteriorating joints. Freitag, et. al. (2019) performed a clinical trial in which a suspension of stem cells in a saline solution were injected into the knee joints of the experimental groups (n=10 individuals over 18 years old with symptomatic knee osteoarthritis received 1 injection of 100 × 10^6 ADMSCs in saline, n=10 individuals received 2 injections of 100 × 10^6 ADMSCs in saline at t=0 and 6 months). The control group (n=10 individuals over 18 years old with symptomatic knee osteoarthritis) received conservative treatment for osteoarthritis. This paper demonstrated that when ADMSCs were injected, they were largely effective in both pain relief (NPRS improvement of 69% from starting point to 12 mo. in both groups) and functional
improvement of the joint for the patients treated (KOOS scale on 1 and 2 injections with subscores for symptom improvement, activities of daily living, sport and recreation, and quality of life both significantly improved when compared to the control), as quantified with numeric pain rating scale, knee injury and osteoarthritis outcome score, and the western Ontario and McMaster universities osteoarthritis index, and MRI imaging (Freitag et. al).
Injectable Hydrogels
Unlike direct injection, a hydrogel provides a material for the stem cells to be embedded and protected from the external environment. This method allows to retain the cells in the site of implantation for a longer period of time, compared to the direct injection of stem cells in a buffer medium. In this latter case, the injected cells are often dispersed in the surrounding aqueous milieu. However, the biomaterial component adds a level of complexity to the system, and extensive characterization of the biomaterials in vitro and in vivo is needed to ensure biocompatibility, and adequate degradation kinetics in order to prevent any immune response or rejection of therapeutics.
One of the hallmarks of a hydrogel is that it can mimic a cell’s natural environment, therefore leading to a healthier, more viable sample or stem cells (Ma Y, et.al). By providing this type of environment for the cells, they are able to proliferate and act in the intended method, due to the biophysical cues provided by the hydrogel. Different types of hydrogels can promote different behaviors, such as adhesion, migration, proliferation, or differentiation, depending on the gel’s characteristics, including degradability, shape memory, hydrophilicity/hydrophobicity (Ma J, et.al).
Additionally, hydrogels can be either natural (taken from the extracellular matrix) or synthetic (artificially manufactured with chemical materials). In general, if a very specific chemical composition is needed or a durable and stable gel is required for the tissue engineering application, a synthetic gel might be best, but if a quickly degrading, non-immune-inducing method is needed, a natural gel may work better (Choe, et.al).
Below is a table organizing various applications of minimally invasive stem cell delivery by way of hydrogels.
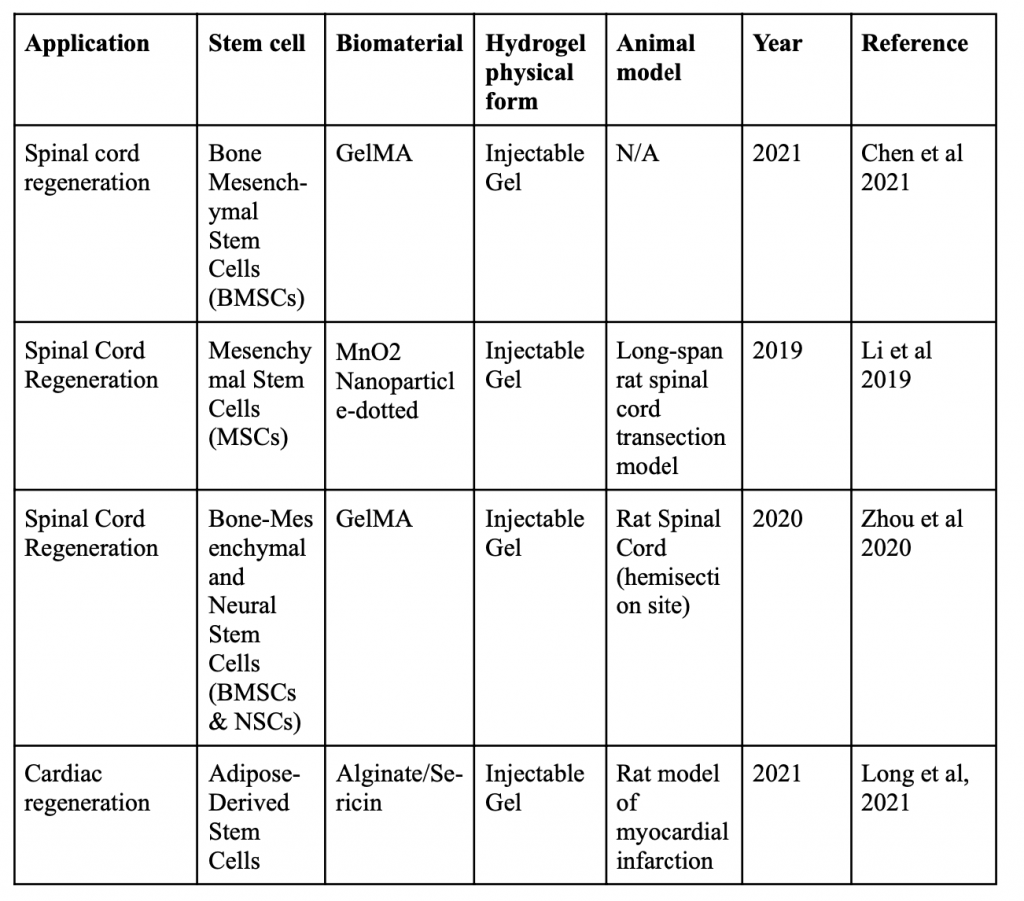
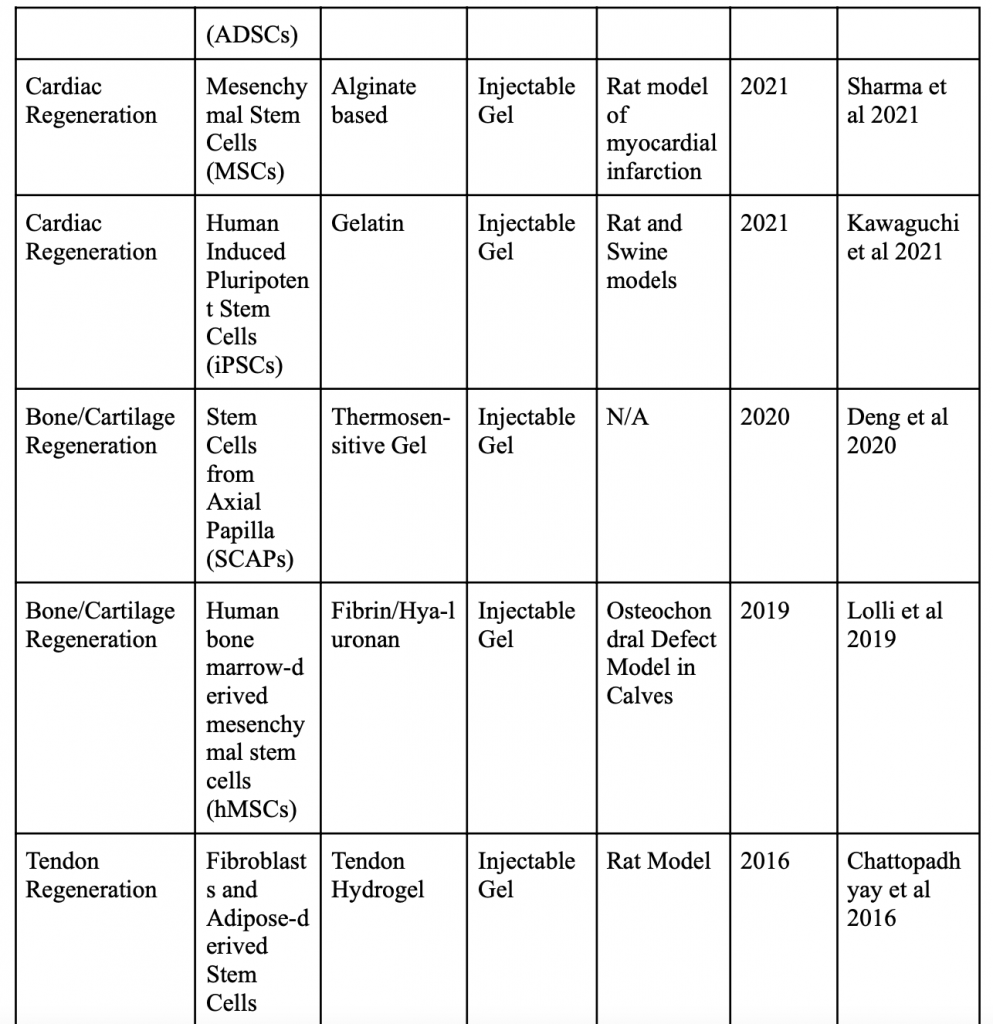
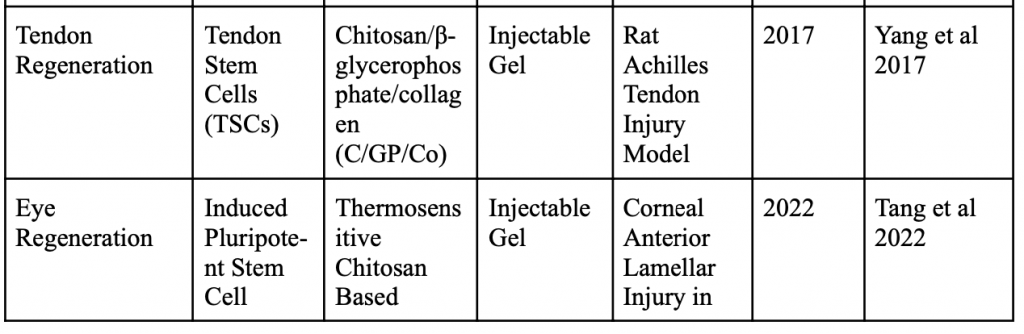
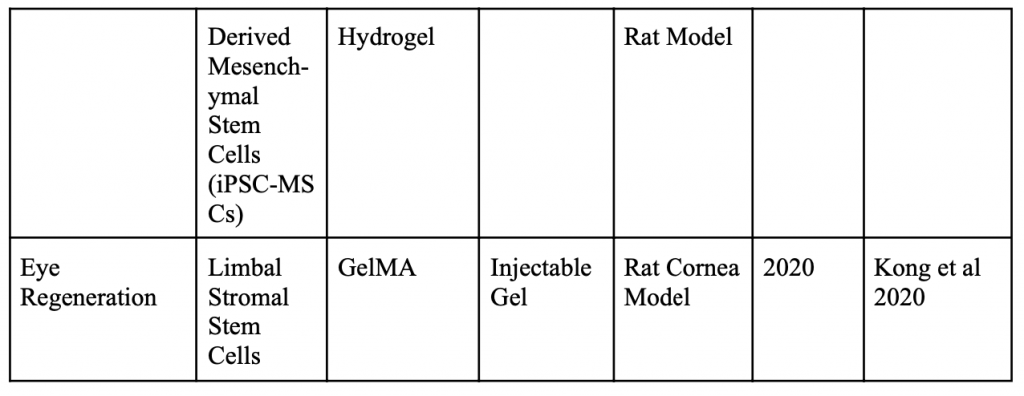
Injectable 3D Scaffold-Free Microtissues
Continuing on, in this methodology of delivery, the cells are cultured in the lab into a 3D spheroid or piece of microtissue, without the use of a supporting biomaterial. There are numerous benefits to this practice in the context of cell protection and proliferation as well. In terms of cell protection, these spheroids/microtissues help greatly in combating damage caused by the pressure induced in the syringe when delivery occurs (Li, et.al). Additionally, these spheroids and microtissues can simulate an in-vivo environment and can be made into different shapes depending on their final location’s needs. This can lead to them being more responsive and productive. In the trials of Li, et. al, 3D microtissues were implanted into mice in a minimally invasive approach, and were trying to deduce whether when the hMSC (human mesenchymal stem cells) would make an impact on necrosis through a mouse limb ischemia model. The results showed that no limb salvage was observed among the control group, but out of those that received the microtissue treatment (density of 10^5 hMSCs), 75% showed salvage, and only 25% resulted in spontaneous limb necrosis (Li, et.al). Moreover, cell sheet technology, which is a type of scaffold-free microtissue, shows promising results. Many times before, when stem cells were just directly injected, the level of cell loss outweighed the benefit of using this method, but with the cell sheet, this problem of cell retention is no longer a problem (Narita et.al). Additionally, because the cell sheets do not involve a scaffold, they do not elicit an immune response, making it safer for the patient. Finally, the work of Yamada, et.al, shows that adipose derived stem cells (ADSCs) show promising results for bone and cartilage regeneration. Due to their rapid regeneration/differentiation rate, they function well for bone and cartilage repair. In this study, the ADSCs were cultured and made into 3D scaffold-free spheroids. They were then implanted (via injection) into rats that had defects in their calvarial bones (Yamada, et. al). Through this study, it was found that the 3D scaffold-free spheroids had lower rates of cell apoptosis compared to ADSC- single cells in both the in-vitro conditions of the laboratory they were manufactured in and in the in-vivo model (for 12 weeks) of the rat. Thus, it was concluded that injectable ADSC-spheroids are a viable option to minimally invasive stem cell delivery for bone and cartilage regeneration (Yamada, et. al).
Conclusion
All together, there are various aspects to delivery of stem cells. Over the course of this review, three important methods of minimally invasive stem cell delivery have been discussed. There are advantages and disadvantages to each. For example, direct injection uses nothing but a saline solution, meaning that it is the most cost-effective way, but it also does not do much in terms of protecting the cells or helping them proliferate; hydrogels can mimic a stem cell’s ideal environment, but deciding between a natural or synthetic hydrogel may prove to be challenging; spheroids and microtissues are the newest method, and are extremely effective since they seamlessly mimic an in-vivo environment, but they have to be grown in a lab first and may not be the most cost-effective. In summary, depending on the need of the research or patient, different methods can be used, but all aspects should be considered to choose the best one.
Citations
1.) Akahane M, Shigematsu H, Tadokoro M, Ueha T, Matsumoto T, Tohma Y, Kido A, Imamura T, Tanaka Y. Scaffold-free cell sheet injection results in bone formation. J Tissue Eng Regen Med. 2010 Jul;4(5):404-11.
2.) Annamalai RT, Hong X, Schott NG, Tiruchinapally G, Levi B, Stegemann JP. Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects. Biomaterials. 2019 Jul;208:32-44.
3.) Asgari N, Bagheri F, Eslaminejad MB, Ghanian MH, Sayahpour FA, Ghafari AM. Dual functional construct containing kartogenin releasing microtissues and curcumin for cartilage regeneration. Stem Cell Res Ther. 2020 Jul 16;11(1):289.
4.) Ashammakhi N, Ahadian S, Darabi MA, El Tahchi M, Lee J, Suthiwanich K, Sheikhi A, Dokmeci MR, Oklu R, Khademhosseini A. Minimally Invasive and Regenerative Therapeutics. Adv Mater. 2019 Jan;31(1):e1804041
5.) Cheng J, Chen Z, Liu C, Zhong M, Wang S, Sun Y, Wen H, Shu T. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomedicine (Lond). 2021 Aug;16(18):1567-1579.
6.) Choe G, Park J, Park H, Lee JY. Hydrogel Biomaterials for Stem Cell Microencapsulation. Polymers (Basel). 2018 Sep 6;10(9):997.
7.) Deng J, Pan J, Han X, Yu L, Chen J, Zhang W, Zhu L, Huang W, Liu S, You Z, Liu Y. PDGFBB-modified stem cells from apical papilla and thermosensitive hydrogel scaffolds induced bone regeneration. Chem Biol Interact. 2020 Jan 25;316:108931.
8.) Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, Paterson K, Boyd R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019 Mar;14(3):213-230
9.) Kawaguchi S, Soma Y, Nakajima K, Kanazawa H, Tohyama S, Tabei R, Hirano A, Handa N, Yamada Y, Okuda S, Hishikawa S, Teratani T, Kunita S, Kishino Y, Okada M, Tanosaki S, Someya S, Morita Y, Tani H, Kawai Y, Yamazaki M, Ito A, Shibata R, Murohara T, Tabata Y, Kobayashi E, Shimizu H, Fukuda K, Fujita J. Intramyocardial Transplantation of Human iPS Cell-Derived Cardiac Spheroids Improves Cardiac Function in Heart Failure Animals. JACC Basic Transl Sci. 2021 Feb 19;6(3):239-254.
10.) Kong B, Chen Y, Liu R, Liu X, Liu C, Shao Z, Xiong L, Liu X, Sun W, Mi S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat Commun. 2020 Mar 18;11(1):1435.
11.) Lee JI, Sato M, Kim HW, Mochida J. Transplantatation of scaffold-free spheroids composed of synovium-derived cells and chondrocytes for the treatment of cartilage defects of the knee. Eur Cell Mater. 2011 Nov 9;22:275-90; discussion 290.
12.) Levin VA, Wright DC, Landahl HD, Patlak CS, Csejtey J. In situ drug delivery. Br J Cancer Suppl. 1980 Apr;4:74-8. PMID: 6932949; PMCID: PMC2149274.
13.) Li L, Xiao B, Mu J, Zhang Y, Zhang C, Cao H, Chen R, Patra HK, Yang B, Feng S, Tabata Y, Slater NKH, Tang J, Shen Y, Gao J. A MnO2 Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano. 2019 Dec 24;13(12):14283-14293.
14.) Li Y, Yan X, Liu W, Zhou L, You Z, Du Y. 3D Microtissues for Injectable Regenerative Therapy and High-throughput Drug Screening. J Vis Exp. 2017 Oct 4;(128):55982.
15.) Lolli A, Sivasubramaniyan K, Vainieri ML, Oieni J, Kops N, Yayon A, van Osch GJVM. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J Control Release. 2019 Sep 10;309:220-230.
16.) Long G, Wang Q, Li S, Tao J, Li B, Zhang X, Zhao X. Engineering of injectable hydrogels associate with Adipose-Derived stem cells delivery for anti-cardiac hypertrophy agents. Drug Deliv. 2021 Dec;28(1):1334-1341.
17.) Ma J, Huang C. Composition and Mechanism of Three-Dimensional Hydrogel System in Regulating Stem Cell Fate. Tissue Eng Part B Rev. 2020 Dec;26(6):498-518.
18.) Ma Y, Lin M, Huang G, Li Y, Wang S, Bai G, Lu TJ, Xu F. 3D Spatiotemporal Mechanical Microenvironment: A Hydrogel-Based Platform for Guiding Stem Cell Fate. Adv Mater. 2018 Dec;30(49):e1705911
19.) Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC, Feng L, Zelka R, Lopez J, Sharma M, Roth S. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019 Mar;197:146-160
20.) Müller P, Lemcke H, David R. Stem Cell Therapy in Heart Diseases – Cell Types, Mechanisms and Improvement Strategies. Cell Physiol Biochem. 2018;48(6):2607-2655
21.) Narita T, Shintani Y, Ikebe C, Kaneko M, Campbell NG, Coppen SR, Uppal R, Sawa Y, Yashiro K, Suzuki K. The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mol Ther. 2013 Apr;21(4):860-7.
22.) Sharma V, Dash SK, Manhas A, Radhakrishnan J, Jagavelu K, Verma RS. Injectable hydrogel for co-delivery of 5-azacytidine in zein protein nanoparticles with stem cells for cardiac function restoration. Int J Pharm. 2021 Jun 15;603:120673.
23.) Tang Q, Lu B, He J, Chen X, Fu Q, Han H, Luo C, Yin H, Qin Z, Lyu D, Zhang L, Zhou M, Yao K. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. 2022 Jan;280:121320.
24.) Toghraie F, Razmkhah M, Gholipour MA, Faghih Z, Chenari N, Torabi Nezhad S, Nazhvani Dehghani S, Ghaderi A. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012 Aug;15(8):495-9.
25.) Yamada Y, Okano T, Orita K, Makino T, Shima F, Nakamura H. 3D-cultured small size adipose-derived stem cell spheroids promote bone regeneration in the critical-sized bone defect rat model. Biochem Biophys Res Commun. 2022 May 7;603:57-62.
26.) Yang Z, Cao H, Gao S, Yang M, Lyu J, Tang K. Effect of Tendon Stem Cells in Chitosan/β-Glycerophosphate/Collagen Hydrogel on Achilles Tendon Healing in a Rat Model. Med Sci Monit. 2017 Sep 27;23:4633-4643.
27.) Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019 Feb 26;10(1):68.
28.) Zhou P, Xu P, Guan J, Zhang C, Chang J, Yang F, Xiao H, Sun H, Zhang Z, Wang M, Hu J, Mao Y. Promoting 3D neuronal differentiation in hydrogel for spinal cord regeneration. Colloids Surf B Biointerfaces. 2020 Oct;194:111214.
About the author

Rishya Gutti
Rishya is currently a senior at Neuqua Valley High School. She is interested in the biological sciences and is an aspiring medical student. Research programs like RISE (Research, Inquiry Skills & Experimentation) have equipped Rishya with the necessary skills to conduct independent research. She is a fourth degree black belt in Taekwondo and has won several national titles in her age group. Rishya also enjoys volunteering her time to teach mathematics to younger students and to promote mental health awareness through a non-profit organization. In her free time, you will find her reading, working out or watching her favorite tv shows.
