Author: Mohan Doddapaneni
Mentor: Dr. Rosalyn Abbott
Moses Brown School
Abstract
The goal of this research is to identify variations of heart murmurs, such as Cardiomyopathy, Aortic Valve Issues, Pulmonary Valve Stenosis, etc., and figure out how to diagnose such murmurs through an ECG. Through using the ECG, and an understanding of what each kind of murmur stems from, conceptual ideas and solutions that are new in the cardiovascular field to heart murmurs can be connected to different conditions, such as tissue printing with alginate and gene editing with CRISPR/Cas9.
Keywords: Brugada Syndrome, Cardiomyopathy, CRISPR/Cas9, FRESH printing, Alginate, ECG, Heart Murmur
Introduction
A heart murmur is caused by turbulent blood flow in the heart valves, or the 4 chambers in the heart, which can be identified by a blowing, wooshing, or rasping sound. A heart murmur is a generalization of a specific issue, much like how the term “illness” can cover everything from stomach bugs and the flu to COVID-19. Murmurs can happen when valves don’t close properly, and blood spews the opposite direction of its path, which is known as regurgitation, or when blood is flowing through a narrowed or stiffened heart valve, which is known as stenosis. Murmurs can be graded on severity, on a scale of 1-6, 1 being the least severe, and 6 being the most severe.
A heart murmur is also characterized by when the heart murmur is occurring. If the blood is not following the directed path when the heart is squeezing out blood, it is a systolic murmur, but if the heart murmur occurs when the heart is filling up with blood, it is a diastolic murmur.
Heart murmurs can range from minor turbulent blood flow, which is not life threatening at all, to severely turbulent blood flow, which could cause the cells in a human body to be malnourished of the nutrients that are required in order for them to function. They can easily be diagnosed through ECGs, which can measure the rate that the heart is pumping blood in different chambers. By being able to identify the severity of the heart murmur, further action can be taken if the heart murmur is too severe. This paper highlights two different solutions– One way is using CRISPR/Cas9 editing. CRISPR can genetically modify cells, which can eradicate hereditary diseases. For example, both dilated and hypertrophic cardiomyopathy, which are hereditary conditions, can be genetically edited with CRISPR/Cas9 such that the disease will not be passed down to further generations. Another way is printing tissue to replace the damaged tissue within the heart through the use of biogels and alginate.
How to Diagnose Heart Murmurs (Cardiomyopathy)
Heart Murmurs are easily distinguished due to their sound. Through the use of stethoscopes, heart murmurs can be detected through “swooshing noises”, which is the sound of the blood moving through the valves and chambers. Another way to distinguish a heart murmur is through an electrocardiogram (ECG). One of the main differences seen in the ECG is the QRS complex. In a normal ECG, a QRS complex is a peak that only travels about 3 units high. In one case of heart murmurs, known as non-ischaemic dilated cardiomyopathy (DCM), the muscles surrounding the ventricles either dilate, or enlarge, making it harder for the heart to pump blood. As a result, the QRS complex is bigger on the ECG. This must mean that the ventricle is not depolarizing properly in DCM.
Another cause of DCM is the Left bundle branch block (LBBB). This occurs in approximately ⅓ of patients found with DCM, and could cause structural changes to the heart. LBBB is diagnosed once the QRS complex’s duration is longer than or equal to 140 ms in duration in a biological male, and 130 ms in a biological female. In a sample taken with 196 patients with DCM, patients with LBBB were found to have greater septal scar burden in the left ventricle, compared to patients who had normal intraventricular conduction. Amiya et al proved that a QRS complex greater than 120 ms was a leading predictor for death/hospitalization due to cardiac issues for patients with non-ischaemic DCM.
Heart Murmurs (Brugada Syndrome)
Brugada Syndrome is an example of a kind of arrhythmia that is inherited. Although it is rare, it is life threatening. It is found in the right bundle branch block (RBBB), and can be seen in the ST segment in leads V1- V3) on an ECG. In about 15-30% of Brugada cases, the gene SCN5A was affected, which resulted in the loss of function of the voltage sodium channels. Calcium and potassium channels as well have also been affected by this.
Types of Heart Murmurs
- Aortic Valve issues
- Mitral Valve issues
- Hypertrophic Cardiomyopathy
- Pulmonary Regurgitation
- Pulmonary Valve Stenosis
- Tricuspid Regurgitation/Stenosis
Current Treatments
Some current treatments are anticoagulants (blood thinners), diuretics (water pills), Angiotensin-converting enzyme (ACE) inhibitors, and beta blockers. All of these treatments directly affect the blood in our system, but there are also experimental treatments that could be used so that medication will be obsolete if properly treated.
Proposed Treatments based on Current Literature
One way is using CRISPR/Cas9 editing. CRISPR can genetically modify cells, which can eradicate hereditary diseases. For example, both dilated and hypertrophic cardiomyopathy is a hereditary condition, but with CRISPR, the gene can be edited such that the disease will not be passed down to further generations.
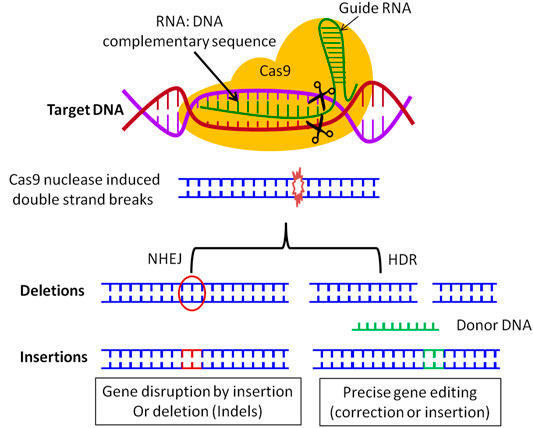
CRISPR was found in Escherichia coli, which is a bacteria. When initially found, there were detections of repeats of DNA, which did not have an identified function. As it was studied, Scientists found the same trait in other microorganisms, and figured out that it was part of the immune system of the bacteria. CRISPR would interact and cut some parts of the DNA of pathogens the Cas Nucleases enzyme. The Cas enzyme is pathogen specific, because of the guide RNA. The RNA stimulates the enzyme, which guides the enzyme to a specific sequence in the DNA.
The CRISPR/Cas9 system needs proto-spacer adjacent motifs (PAMs), which are 2-6 base pair sequences (Adenosine paired with Thymine, Guanine paired with Cytosine) that are in the virus genome, that are similar to Cas nucleases targeted sequences. The CRISPR/Cas9 sequence can guide the RNA to a precise location in the destination genome, modify it, edit current genes, deliver new genes, knock out or knock in the gene, or delete the gene entirely.
The CRISPR/Cas9 system can be applied to certain hereditary heart diseases and murmurs. It must be done on fetuses, however, since fetuses have fewer cells than fully developed adults or children, and will have the most effective results. In about 15-30% of Brugada cases, the gene SCN5A was affected, which resulted in the loss of function of the voltage sodium channels. Calcium and potassium channels as well have also been affected by this. Without functioning sodium, calcium, and potassium channels, the heart will not contract properly, the muscle walls could dilate, and the heart could fail. The SCN5A gene contains the code for the alpha subunit of the main cardiac sodium channel Nav1.5, which is responsible for the normal function of inward sodium current (INa). Variants of the gene SCN5A could cause abnormal function of INa, which means the heart would not be able to depolarize quickly, which would cause a cascading effect that results in the electrical signals within the heart to fail. By using CRISPR/Cas9 editing, scientists could hypothetically isolate the affected sequence in the SCN5A variant, and use CRISPR to edit the gene so that the sodium channels work again. By doing this, hypothetically, any hereditary heart disease could be cured, ending the heart disease in future generations.
Another solution is through tissue printing. As stated before, LBBB causes septal scar tissue in the LV, so a possible solution would be to surgically remove the scar tissue and replace it with printed tissue. We can use techniques such as FRESH (Freeform Reversible Embedding of Hydrogels) to print the tissue in the septum of the heart. At Carnegie Mellon University, Adam Feinberg utilized FRESH and bioprinted a 3D model of a human heart. Through using alginate, Feinberg was able to replicate most of the properties of the heart tissue.
Through using alginate and FRESH, and MRI data of patients, scientists could print the septal tissue in the heart. Surgeons can remove the scar tissue in the septum, and replace it with the alginate tissue, which would be an exact replica of the patient’s septum tissue.
Some issues that could arise is the structural integrity of the alginate tissue. The tissue is still undergoing development, as it is not the strongest it could potentially be. With the strength of the blood flow in the heart, the blood pressure could damage the tissue, which could create serious heart issues. Some solutions that were introduced were using collagen with the alginate. Through introducing this protein, the tissue could become stronger.
Conclusion
Heart murmurs and other cardiovascular diseases affect many people around the world. Tissue printing and CRISPR/Cas9 editing are only a few ways of many conceptual ideas that many different heart diseases and issues, such as Cardiomyopathy and Brugada’s syndrome, can be treated. Through the use of ECGs, many different kinds of heart diseases can be identified, and with these treatments, many hereditary and non-hereditary heart diseases can be treated/cured.
Acknowledgements
I would like to thank Professor Rosayln Abbott of Carnegie Mellon University for her guidance and support throughout the process of writing this research paper.
References
[1] Abdelnour, Sameh A., et al. “The Potential of CRISPR/Cas9 Gene Editing as a Treatment Strategy for Inherited Diseases.” Frontiers in Cell and Developmental Biology, vol. 9, 15 Dec. 2021, https://doi.org/10.3389/fcell.2021.699597.
[2] “The Cardiac Action Potential.” ITACA-CM, www.itaca.edu.es/cardiac-action-potential.htm#:~:text=The%20phases%20of%20the%20cardiac,the%20duration%20potential%
20ventricular%20action. Accessed 16 Nov. 2023.
[3] Carroll, Dan. “3D Bioprinted Heart Provides New Tool for Surgeons.” Carnegie Mellon University, 10 Dec. 2020, www.cmu.edu/news/stories/archives/2020/december/3d-bioprinted-heart.html. Accessed 16 Nov. 2023.
[4] Chen, Michael A., and David C. Dugdale, editors. “Heart Murmur.” Penn Medicine, www.pennmedicine.org/for-patients-and-visitors/patient-information/conditions-treated-a-to-z/heart-murmur. Accessed 14 Nov. 2023.
[5] Crescenzi, Cinzia, et al. “The Electrocardiogram in Non-ischaemic-dilated Cardiomyopathy.” European Heart Journal Supplements, vol. 25, no. Supplement_C, 26 Apr. 2023, pp. C179-C184, https://doi.org/10.1093/eurheartjsupp/suad043.
[6] “Heart Murmur.” Yale Medicine, www.yalemedicine.org/conditions/heart-murmur. Accessed 14 Nov. 2023.
[7] Lee, A., et al. “3D Bioprinting of Collagen to Rebuild Components of the Human Heart.” Science, vol. 365, no. 6452, 2 Aug. 2019, pp. 482-87, https://doi.org/10.1126/science.aav9051. Accessed 16 Nov. 2023.
[8] Li, Wenjia, et al. “SCN5A Variants: Association with Cardiac Disorders.” Frontiers in Physiology, vol. 9, 9 Oct. 2018, https://doi.org/10.3389/fphys.2018.01372.
[9] Makarova, Kira S., et al. “A Putative RNA-interference-based Immune System in Prokaryotes: Computational Analysis of the Predicted Enzymatic Machinery, Functional Analogies with Eukaryotic RNAi, and Hypothetical Mechanisms of Action.” Biology Direct, vol. 1, no. 1, 16 Mar. 2006, https://doi.org/10.1186/1745-6150-1-7. Accessed 16 Nov. 2023.
[10] Sattar, Yasar, and Lovely Chhabra. “Electrocardiogram.” National Library of Medicine, www.ncbi.nlm.nih.gov/books/NBK549803/#:~:text=A%20regular%20rhythm%20ECG%20
has,activation%20from%20the%20SA%20node. Accessed 16 Nov. 2023.
[11] Takahashi, Kiichi, et al. “Detection of Pathologic Heart Murmurs Using a Piezoelectric Sensor.” Sensors, vol. 21, no. 4, 16 Feb. 2021, p. 1376, https://doi.org/10.3390/s21041376. Accessed 16 Nov. 2023.
About the author

Mohan Doddapaneni
Mohan is a student at Moses Brown School, located in Providence, Rhode Island. He has lived in Bellingham, Massachusetts since he was a small child. He s a strong passion for Biology, and hopes to become a doctor one day. He loves to play music, is part of the schools Jazz Band and Wind Ensemble, and swims for the Moses Brown Swim Team.