
Author: Youran Wang
Beijing No.4 High School International Campus
Abstract
The environmental pollution is threatening not only humans themselves but also the ecosystem. A wide variety of environmental pollutants directly or indirectly affect DNA, causing genotoxicity of wild species. Numerous studies reveal that the wild species can be critical bio-indicator of environmental pollution, including snails. So far, all the related works are carry out with laboratory bred snails in a field environment or wild snails in a laboratory condition. The researcher hypothesizes that using the free-living snails will offer an appealing approach for in situ environmental biomonitoring of the sampling location. In this study, all results indicated a significantly positive correlation between the airborne pollutants and DNA damage from the samples of wild-caught land snail Cathaica fasciola. It strongly suggests that the wild snail Cathaica fasciola can be used as a novel in situ bioindicator in air pollution monitoring of their habitat.
Keywords: Bioindicator, Snail, Genotoxicity, Comet assay, Airborne pollutants
Introduction
Environmental pollution is one of the major challenges in modern human society, especially in China(Liu et al., 2016). It is threatening not only humans themselves but also the ecosystem. The environmental contaminants impact the mutagenic and gene-toxicity (Sandhu and Lower, 1989). Thus, wild species in a natural environment can be critical approaches to study environmental pollution regarding the use of animals or plants as bioindicators(Azzazy, 2020). So far, numerous studies reveal that bioindicators are vital in terms of monitoring pollutants and essential for protecting the ecosystem stability and human health(Carignan and Villard, 2002).
Genotoxicity is considered as one of the most important toxic endpoints because a wide variety of environmental pollutants directly or indirectly affect DNA(Costa, 2022). The single cell gel electrophoresis or comet assay is one of the most sensitive approaches to detect a broad spectrum of primary DNA damage as the single-strand breaks or the double-strand breaks, and alkali-labile sites and cross-links of different cell types from different species, including snails(de Souza et al., 2015; Sargsyan et al., 2022; Singh et al., 1988).
Different experiments demonstrate that snails offer a critical approach for environmental biomonitoring(de Souza et al., 2015; Dhiman and Pant, 2021; Sargsyan et al., 2022). So far, all the related works are carried out with laboratory bred snails in a field environment or wild snails in a laboratory condition(Dhiman and Pant, 2021; Ianistcki et al., 2009; Zheng et al., 2013). As snails are widely distributed throughout the world and easy to acquire, the researcher hypothesizes that using free-living snails in their natural habitat as environmental monitoring. Importantly, free-living snails will offer an appealing approach for environmental biomonitoring of the sampling location. Studies need to conduct to reveal this hypothesis.
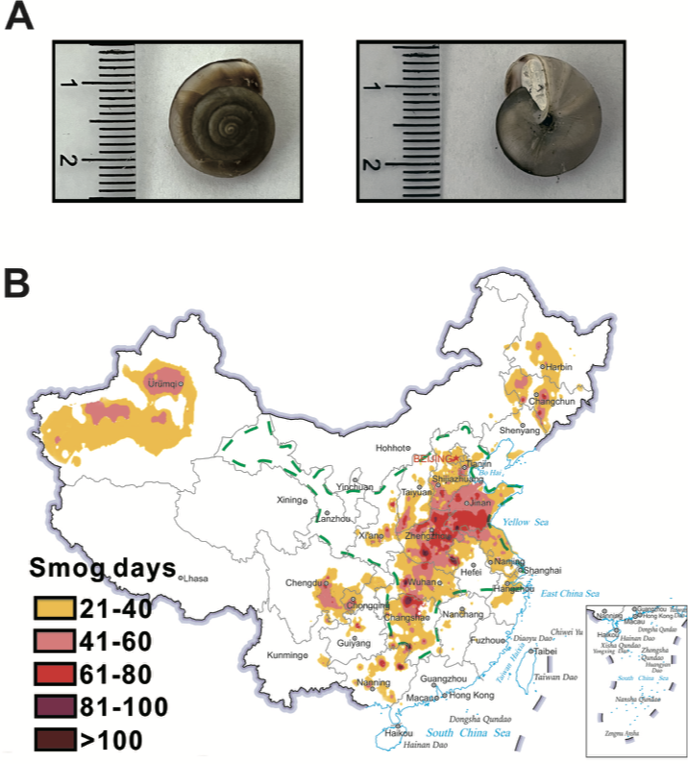
The Cathaica fasciola (Draparnaud, 1801) is an land snail with a wild distribution in northern China (Dong et al., 2020)(Fig. 1b). The main goal of this work aims to investigate the DNA damage by using comet assay with the wild-caught land snail Cathaica fasciola in their habitat as a naturally occurring bioindicator of airborne pollutants.
In this study, the snails have been sampled in the field from various regions of Beijing and the comet assay has been carried out to study the effect of the air pollution on DNA damage. All results indicated a significantly positive correlation between DNA damage and the airborne pollutants from the samples of Cathaica fasciola. It strongly suggests that the free-living snail Cathaica fasciola can be used as a novel in situ bioindicator in air pollution monitoring of their habitat.
Materials and methods
Study area
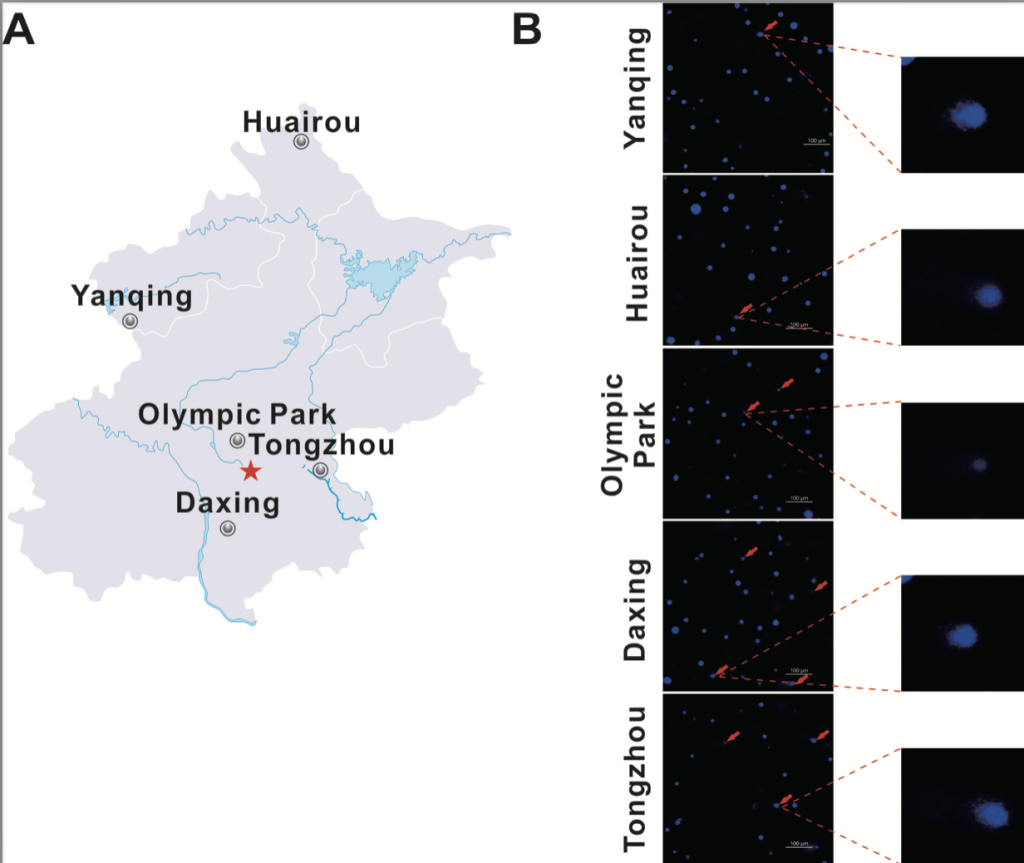
Five different locations were selected for the sampling of snails. Snails were captured in Beijing, China from Huairou (41◦31′52′′N and 116◦39′41′′E), Yanqing (40◦26′08′′N and 115◦54′37′′E), Olympic park (40◦00′06′′N and 116◦23′22′′E), Daxing (39◦40′14′′N and 116◦20′43′′E) and Tongzhou (39◦52′574′′N and 116◦39′34′′E). All locations represented on the map in figure 2a.
Snail collection
In total 10 individuals of Cathaica Fasciola (Fig. 1a) with a similar shell size 17x14mm and weighing 0.9 g were collected at each site during October, 2022. The snails were submitted for analysis on the following day.
Alkaline comet assay
The alkaline comet assay was performed according to the method of Singh et al. with several modifications(Singh et al., 1988). Briefly, snails were dissected, and digestive glands were freshly dissected out. The tissue was homogenized with 1mL of ice-cold 0.05M phosphate buffer, pH 7.4 and filtered with a cell strainer (70um, BD Falcon). The filtrate was centrifuged at 500g for 10minutes to pellet the digestive gland cells. The cell pellets were added to 80 uL of 0.8% (w/v) low melting point (LMP) agarose. The mixture was then layered on slides precoated with 0.8% (w/v) normal melting point agarose and covered with a cover glass and solidified at 4°C for 10 min. A final layer of 80 uL of 0.8% (w/v) LMP was added to the slides, which were again covered with a cover glass. Following solidifications at 4°C, the cover glasses were removed and the slides were ready for the assay.
The slides were immersed in freshly prepared lysing solution (2.5 M NaCl, 100mM Na2EDTA·2H2O, 10 mM Tris, pH 10, with 1% Sodium lauroyl sarcosinate, 10% DMSO, and 1% Triton X-100) for 1 hour at 4°C. Upon completion of lysis, slides were immersed in alkaline electrophoresis buffer (300 mM NaOH and 1 mM Na2EDTA·2H2O) for 20 min at 4°C to allow DNA unwinding. Subsequently, the electrophoresis was conducted at 4°C for 20 min (25 V and 300 mA), using a Bio-Rad power supply. After electrophoresis, the slides were neutralized in 0.4 M Tris buffer at pH 7.5 and stained with DAPI (Vector Labs). Fluorescence was observed under a laser scanning confocal microscopy (Nikon).
Data analysis
To evaluate the damage frequency, at least 400 randomly selected cells from each slide were counted. The tail DNA percentage was calculated for each sample based on the number of nuclei with tail versus the total nuclei.
Results
In recent years, China is facing severe air pollution problems which is more critical to the north China plain, including Beijing(Liu et al., 2016) (Fig. 1b). Cathaica fasciola is a native snail widely distributed in China(Dong et al., 2020). The distribution of Cathaica fasciola covers that of heavy air pollution area in the north China plain(Dong et al., 2020) (Fig. 1b). Taken together with its easy to identify and sampling, the researcher supposed that the wild snail of Cathaica fasciola could be used for biomonitoring airborne pollutants in regions of north China plain.
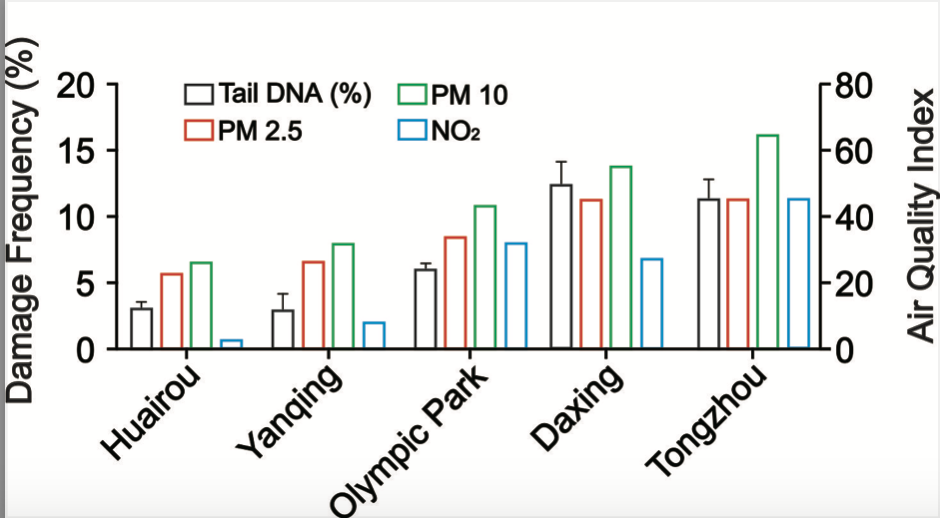
To evaluate the usage of Cathaica fasciola as a potential in situ biomonitoring tool, the researcher sampled individuals of Cathaica fasciola from five different locations in Beijing. Huairou and Yanqing, which are in the ecological conservation areas, locate about 100 km from the urban area of Beijing (Fig. 2a). Olympic park, Tongzhou and Daxing are three urban sites (Fig. 2a). The DNA damage was detected by alkaline comet assay following immunofluorescence staining with DAPI. As shown in Figure 2b, tail DNA were obviously visible in the images (red arrow). Damage frequency (% tail DNA) were significantly higher for samples from Tongzhou (11.5%) and Daxing (12.5%) than that in Huairou (3.4%) and Yanqing (3.1%) (Fig. 3). It was observed a damage frequency of 6.7% in the sample from Olympic park (Fig. 3).
Figure 3 illustrates the annual average spatial distribution of particulate matter (PM2.5, PM10) and nitrogen oxides (NO2) found in the five snail sampling locations. Notably, the annual mean index of PM2.5 and PM10 showed a significant positive correlation between DNA damage frequency. From the given figure, it is observed that Olympic park showed a higher annual mean index of NO2 than Daxing (32 vs 28). Whereas, DNA damage frequency showed the exact opposite trend (6.7% vs 12.5%). Tongzhou took the higher NO2 index than Daxing (33 vs 28). However, the DNA damage frequency showed similar levels (11.5% vs 12.5%). Above results indicated that DNA damage frequency is strongly correlated with concentration of particulate matter and showed a moderate relationship with NO2 concentration.
Discussion
To my knowledge, this is the first application of land snails of Cathaica fasciola that were sampled in the field for studying the genotoxicity of airborne pollutants. Fundamentally, my studies prove that the wild snail of Cathaica fasciola could be used as a reliable and low-cost bioindicator of airborne pollutants. Importantly, habitats of Cathaica fasciola overlap with the areas most affected by the haze in China on which indicate a broad scope of application.
The results indicated that DNA damage frequency is strongly correlated with particulate matter (PM) concentration but not NO2. Indeed, studies have confirmed that metal(loid)s exhibits different genotoxicological mechanisms to land snails (Louzon et al., 2021). According to the World Health Organization (WHO), air pollutants with the strongest evidence for public health concern include PM, carbon monoxide (CO), ozone (O3), NO2 and sulfur dioxide (SO2). A comparative research needs to be carried out to better assess the genotoxicity risks from various air contaminants.
Wild snail traits are stronger related to natural conditions(Dong et al., 2020). This makes the evaluation of wild snail experiments much more complex than experiments with bred laboratory snails. So far, this work has been calibrated only with samples from five locations. To improve the validation of this work, I intent to expand the range of sample sites, especially in the most haze-affected areas.
Declaration of Competing Interest
The author declare that there are no known competing interests.
References
Azzazy, M.F. (2020). Plant bioindicators of pollution in Sadat City, Western Nile Delta, Egypt. PLoS One 15, e0226315.
Carignan, V., and Villard, M.A. (2002). Selecting indicator species to monitor ecological integrity: a review. Environ Monit Assess 78, 45-61.
Costa, P.M. (2022). Current aspects of DNA damage and repair in ecotoxicology: a mini-review. Ecotoxicology 31, 1-11.
de Souza, M.R., da Silva, F.R., de Souza, C.T., Niekraszewicz, L., Dias, J.F., Premoli, S., Correa, D.S., Soares Mdo, C., Marroni, N.P., Morgam-Martins, M.I., et al. (2015). Evaluation of the genotoxic potential of soil contaminated with mineral coal tailings on snail Helix aspersa. Chemosphere 139, 512-517.
Dhiman, V., and Pant, D. (2021). Environmental biomonitoring by snails. Biomarkers 26, 221-239.
Dong, Y.J., Wu, N.Q., Li, F.J., Zhang, D., Zhang, Y.T., Huang, L.P., Chen, X.Y., Wu, B., and Lu, H.Y. (2020). Anthropogenic modification of soil communities in northern China for at least two millennia: Evidence from a quantitative mollusk approach. Quaternary Sci Rev 248.
Ianistcki, M., Dallarosa, J., Sauer, C., Teixeira, C.E., and da Silva, J. (2009). Genotoxic effect of polycyclic aromatic hydrocarbons in the metropolitan area of Porto Alegre, Brazil, evaluated by Helix aspersa (Muller, 1774). Environ Pollut 157, 2037-2042.
Liu, J., Mauzerall, D.L., Chen, Q., Zhang, Q., Song, Y., Peng, W., Klimont, Z., Qiu, X., Zhang, S., Hu, M., et al. (2016). Air pollutant emissions from Chinese households: A major and underappreciated ambient pollution source. Proc Natl Acad Sci U S A 113, 7756-7761.
Louzon, M., Gimbert, F., Belly, T., Amiot, C., Pauget, B., de Vaufleury, A., and Capelli, N. (2021). From environmental bioavailability of metal(loid)s to their ecogenotoxicological effects in land snails. Environ Sci Pollut Res Int 28, 43629-43642.
Sandhu, S.S., and Lower, W.R. (1989). In situ assessment of genotoxic hazards of environmental pollution. Toxicol Ind Health 5, 73-83. 6
Sargsyan, A., Hovhannisyan, G., Simonyan, A., Arakelyan, M., Arzumanyan, M., and Aroutiounian, R. (2022). Application of land snail Helix lucorum for evaluation of genotoxicity of soil pollution. Mutat Res Genet Toxicol Environ Mutagen 878, 503500.
Singh, N.P., McCoy, M.T., Tice, R.R., and Schneider, E.L. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175, 184-191.
Zheng, S., Wang, Y., Zhou, Q., and Chen, C. (2013). Responses of oxidative stress biomarkers and DNA damage on a freshwater snail (Bellamya aeruginosa) stressed by ethylbenzene. Arch Environ Contam Toxicol 65, 251-259.
Figure legend
Fig. 1. The land snail of Cathaica Fasciola distributes in the haze-affected areas in the north China plain.
a. Cathaica Fasciola (Draparnaud, 1801). b. The region surrounded by green dash lines indicate the distribution areas of the land snail of Cathaica Fasciola. The average number of smoggy days nationwide are presented according to the 2021 meteorological bulletin of atmospheric environment, which released by China meteorological administration.
Fig. 2. Comet assay shows DNA damage in the digestive gland cells of Cathaica Fasciola.
a. Map of the study area. The gray circle indicates the sampling sites of snails. The red star indicates the Beijing city center. b. Comet images of the digestive gland cells stained with DAPI and observed under a fluorescent microscope. Red arrows points out the tails, which indicate DNA damage. Representative images are presented. High magnification is presented on the right.
Fig. 3. DNA damage frequency is significant positively correlated with airborne pollutants.
Quantitative comet assay results in digestive gland cells are presented. Data are the mean ± SEM (n= 10). According to the Beijing ecology and environment statement 2021, the annual mean concentration of PM2.5, PM10 and NO2 of the five different sampling sites are shown in the chart.
About the author

Youran Wang
Jasmine Wang is an 11th grade student at Beijing No.4 High School International Campus. She is passionate about sociology and child education and is willing to speak out about the inequalities in education. She is the proprietor of DaoXue Club which is a volunteering constitution that includes free teaching classes for blind students. Meanwhile, she is fond of photographing and expects the photographs can become a realistic reflection of social problems, be worthy of careful consideration, and contribute to the solution of these problems.
