Author: Yicheng Ding
Shanghai Pinghe Bilingual School
Abstract
Mechanical forces are important regulators of cellular metabolism. However, the molecular regulation of mechanotransduction remains unclear except for bone homeostasis and osteoblast differentiation. Here, I identified the mechanosensitive channels PIEZO1 and PIEZO2 as key regulators of hepatocellular carcinoma growth and migration. Bioinformatics from the GEPIA database indicates that high level of PIEZO1/2 TPM associates with the HCC tissue while there is a comparatively low level of PIEZO1/2 in adjacent tissues. With cultured LM3 cells in DMSO solutions, the inhibition of PIEZO1/2 by Dooku1 led to diminished proliferation and declined transformation of hepatocellular carcinoma cells, while the promotion of PIEZO1/2 by Yoda1 resulted in enhanced LM3 cells proliferation and metastasis. Cell counting Kit-8 assay and transwell migration assay were separately performed to measure the number of HCC cells grown and migrated. Collectively, these findings suggest that PIEZO1/2 has a regulatory role in HCC development, which can serve as an innovative therapeutic target for prognosis and treat cancer.
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent malignant tumor and the fourth leading cause of cancer death worldwide, and China is one of the most high-risk HCC areas [1]. Risk factors for HCC include chronic hepatitis B and hepatitis C, alcohol addiction, metabolic liver disease, and exposure to dietary toxins such as aflatoxins and aristolochic acid. All of these risk factors are potentially preventable, emphasizing the importance of risk reduction in palliating the global burden of HCC; nonetheless, even in nations with adequate medical resources, HCC surveillance is significantly underutilized [2]. Despite various treatments like chemotherapy and immunotherapy, myriads of cancer patients suffered from relapse problems as well as serious side effects, cytokine release syndrome (CRS), and neurotoxicity for example. According to the International Agency for Research on Cancer, there was an estimated 9.9 million cancer deaths in 2020, with HCC accounting for over 11% of all cancer fatalities (Globocan, 2020). This called for a strong need for immunologists and biochemists to work out a better therapeutic solution.
Since 2020, researchers started to focus on extracellular matrix (ECM) that contributes to intense interaction with HCC for tumorigenesis, proliferation, migration, and immune invasion [3]. In 2021 David Julius and Ardem Patapoutian discovered thermal transducers TRPV1 and TRPM8. By using a functional screen of candidate genes expressed in a mechanosensitive cell line, PIEZO1 and PIEZO2, two mechanically activated ion channels, were discovered and proved to represent an entirely new class of ion channels that function as mechanical sensors [4]. They transmit chemical signal through a plug-and-latch mechanism, with a structure of thirty-eight transmembrane units categorized as blade, beam, and anchor. ECM stiffening-induced mechanical stimuli, as well as extracellular matrix itself, can activate cell membrane receptors and mechanosensors such as PIEZO1 and TRPV1 to modulate the malignant phenotype of HCC. Following studies demonstrated that a lack of PIEZO1 produced anomalous blood vessel development, while a mutation in PIEZO1 induced increased calcium influx, resulting in decreased erythrocyte capacity. Besides their strong correlation with heart rate and respiration, Piezo1/2 expression was demonstrated to be significantly up-regulated in HCC tissues and cell lines, which is associated with aggressive pathological features and poor prognosis. Intrigued by these researches, I conducted a series of experiments to investigate whether the presence of Dooku1, a selective antagonist of the endogenous Piezo1 channel, would have an impact on HCC and paired normal tissue growth.
In this paper, I report the discovery of the promoting and suppressive effects of mechanosensitive ion channels PIEZO1/2 on proliferation and transformation of hepatocellular carcinoma. When PIEZO1/2 agonist Yoda1 is utilized against the mechanical channel, the cultured HCC cells have higher growth and stronger metastasis ability compared with the control group, while when the antagonist Dooku1 is employed, the cultured HCC cells demonstrate lower growth and weaker potential of transformation than the DMSO group. Collectively, these qualitative observations manifest the causal relationship between mechanical force receptor PIEZO1/2 and HCC growth and migration, which indicates a new possible therapeutic target for drug design and other treatments that suppress HCC proliferation and metastasis.
Results
Expression of PIEZO1/2 in HCC and adjacent tissue and different survival rates
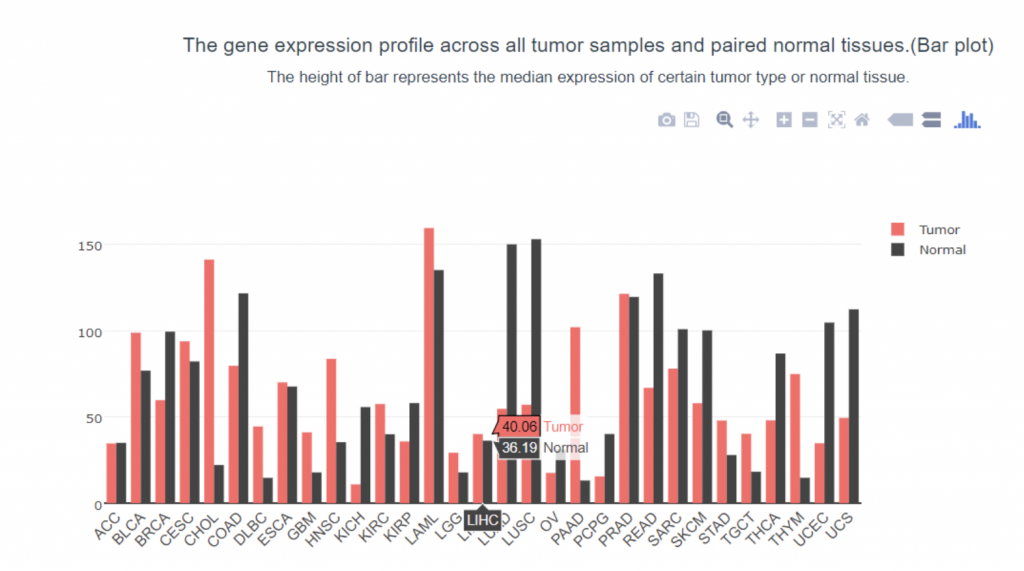
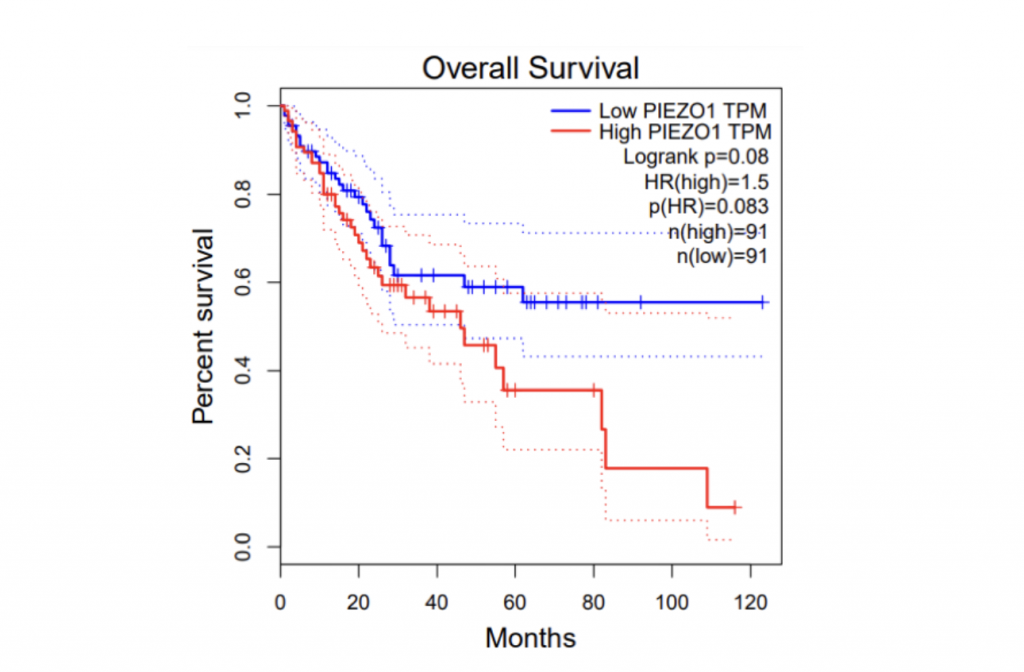
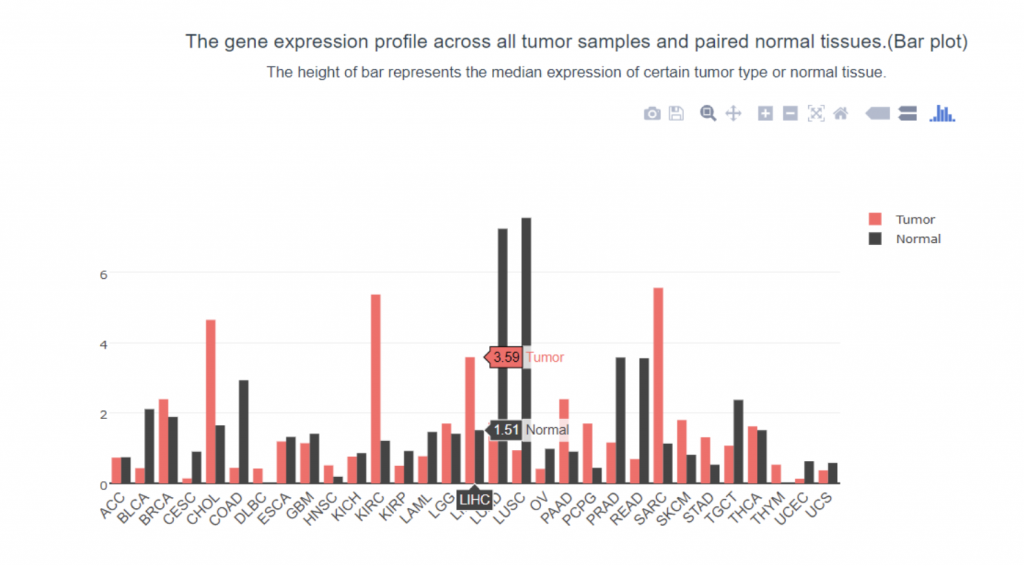
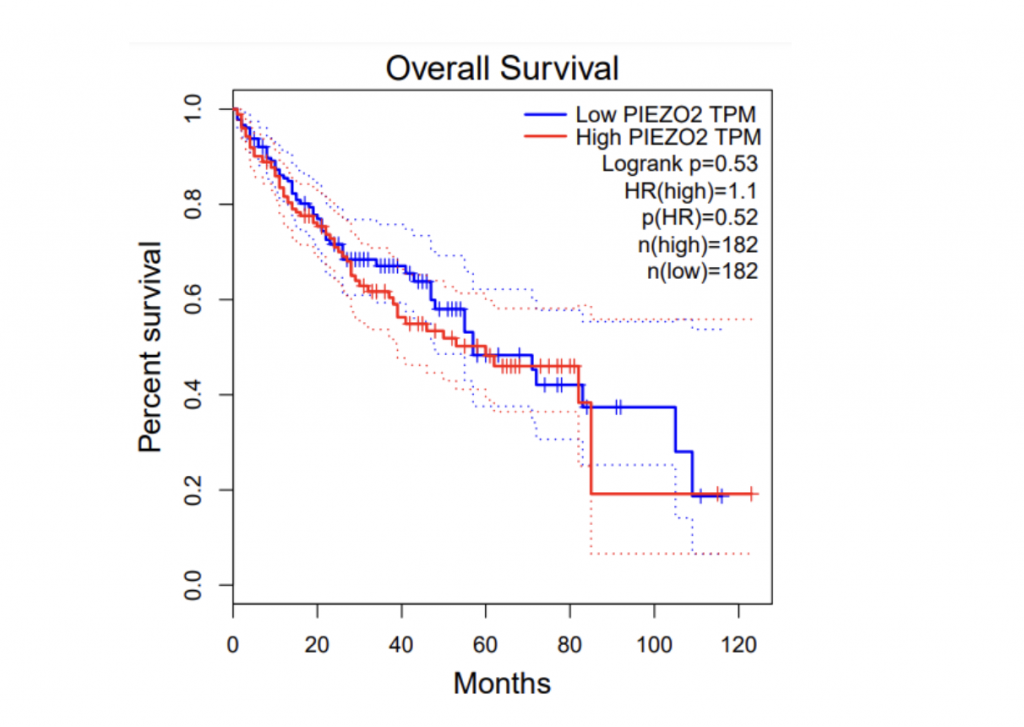
To determine if PIEZO1/2 plays any role in HCC development, I first examined the expression profiles of PIEZO1/2 in the commonly used HCC cells LM3 by using bioinformatics obtained from database GEPIA and TCGA. The expression of PIEZO 1 in HCC and para-carcinoma tissue do not have a significant difference: 10.7% higher expression in HCC than adjacent tissues, while the expression of PIEZO 2 in HCC is 138% considerably higher than the surrounding tissue. This suggests a possible correlation between PIEZO1/2 and HCC development. Then I investigated the survival rates of HCC patients with variation in PIEZO1/2 expression. It turned out that there is a general trend between high PIEZO1/2 expression and low survival rate. Among the 182 HCC patients from GEPIA database, 73% of HCC patients with low PIEZO1 transcripts per million (TPM) survived and 65% of HCC patients with high PIEZO1 TPM survived after 20 months of HCC development. After 90 months of HCC illness, 57% of patients with low PIEZO1 TPM were still alive while only 19% of patients with high PIEZO1 TPM survived. PIEZO2 has similar scenarios of survival ratio with PIEZO1: 37% of patients with low PIEZO2 TPM survived and after 90 months of HCC development. Collectively, these information promoted me to further examine whether mechanical channel has a direct impact on HCC cell either growth or migration.
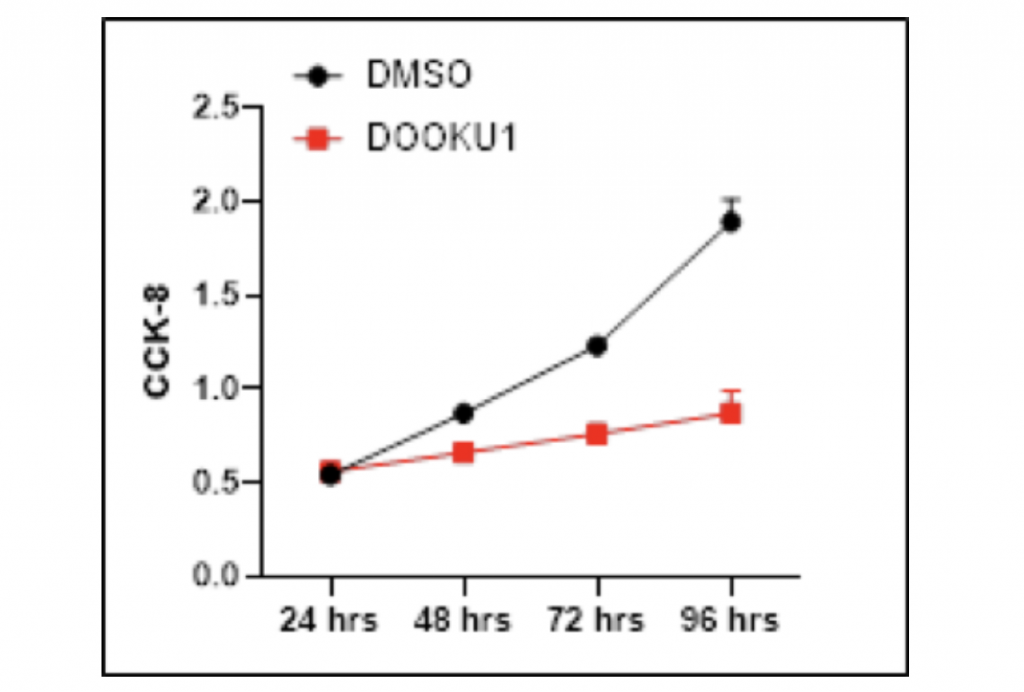
Proliferation of HCC cells is decreased after the addition of Dooku1
To confirm the causal relationship between PIEZO1/2 expression and HCC cells growth, I manipulated the mechanosensitive channels by utilizing Dooku1 as an antagonist that inhibits PIEZO1/2 expression. The HCC LM3 cells samples were separately grown in DMSO with and without Dooku1 molecules as the experimental and control group. After 96-hours growth period, the quantity of HCC cells were measured by Cell Counting Kit-8. Remarkably, the addition of Dooku1 antagonist in HCC cells culture solution decreased the cell growth compared with the results from the DMSO vector. After 48-hours proliferation time, the amount of HCC cells grown with Dooku1 was 24.1% fewer than the control group. The inhibition effect magnified when the growth period went longer as the visual difference between two lines diverge increasingly further away. After 96-hours proliferation time, the growth of experimental group was 54.0% fewer than the vector group. There is one anomaly data where the growth of HCC cells with Dooku1 after 24 hours is 3.7% more than that of control group. One possible explanation is that the Dooku1 molecules added into DMSO solution had not fully dissolved and performed its role of inhibiting PIEZO1/2 channels. Also due to Dooku1’s analogous shape with Yoda1 and incomplete solvation, PIEZO1/2 functioning may be accidentally reinforced and therefore stimulated the HCC cells growth.
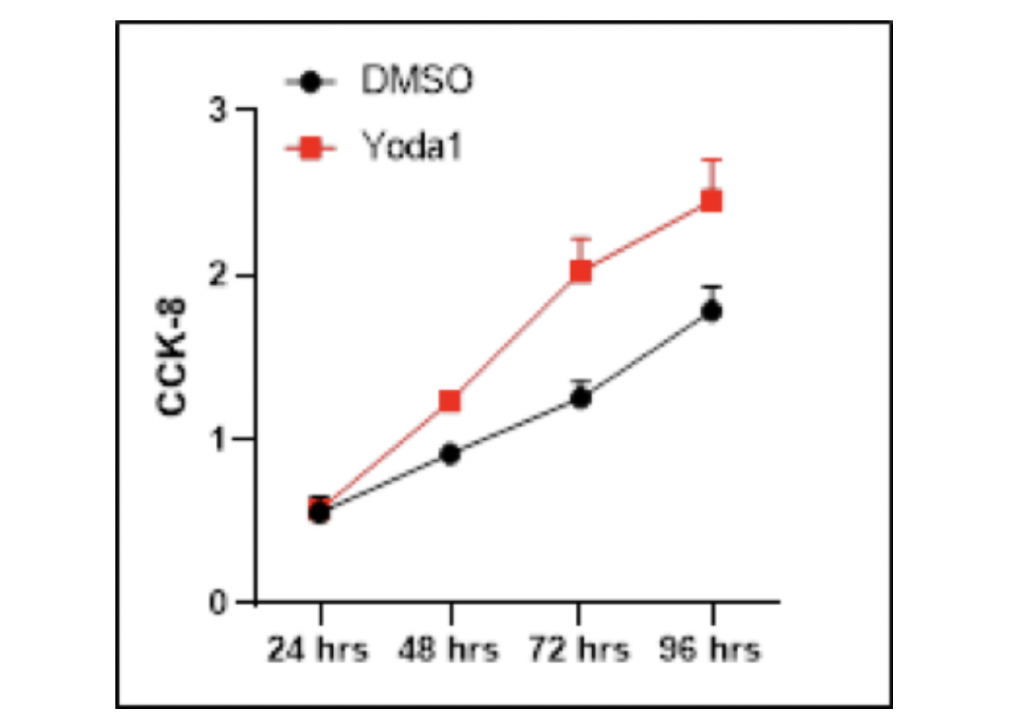
Accretion of HCC cells is increased after the utilization of Yoda1
The inhibitory effect of Dooku1 antagonist on HCC cells further inspired me to investigate whether Yoda1, the agonist of mechanical channel PIEZO1/2, could result in a stimulating effect on HCC proliferation. Similarly, HCC LM3 cells were cultured in DMSO separately with and without Yoda1 as the experimental and control groups. Cell Counting Kit-8 was used to count the number of HCC cells after 96 hours of growth. Noticeably, the addition of Yoda1 molecules significantly promoted the growth of HCC cells in comparison with the vector group, and the difference between DMSO and Yoda1 became increasingly distinct as growth period went longer. After 24-hours proliferation time, the amount of HCC cells grown with Dooku1 was only 0.02 more than the control group. However, after 48-hours proliferation time, the growth of experimental group had become 35.2% higher than the vector group, while after 72-hours growth period, this percentage difference amplified to 61.6%. If the experiment were conducted with longer duration, the growth-promoting effect could be more prominent. In contrast to Dooku1, there was not any outliers that failed to follow the general trend. Taken together, these data suggest that Yoda1 stimulates HCC cell proliferation.
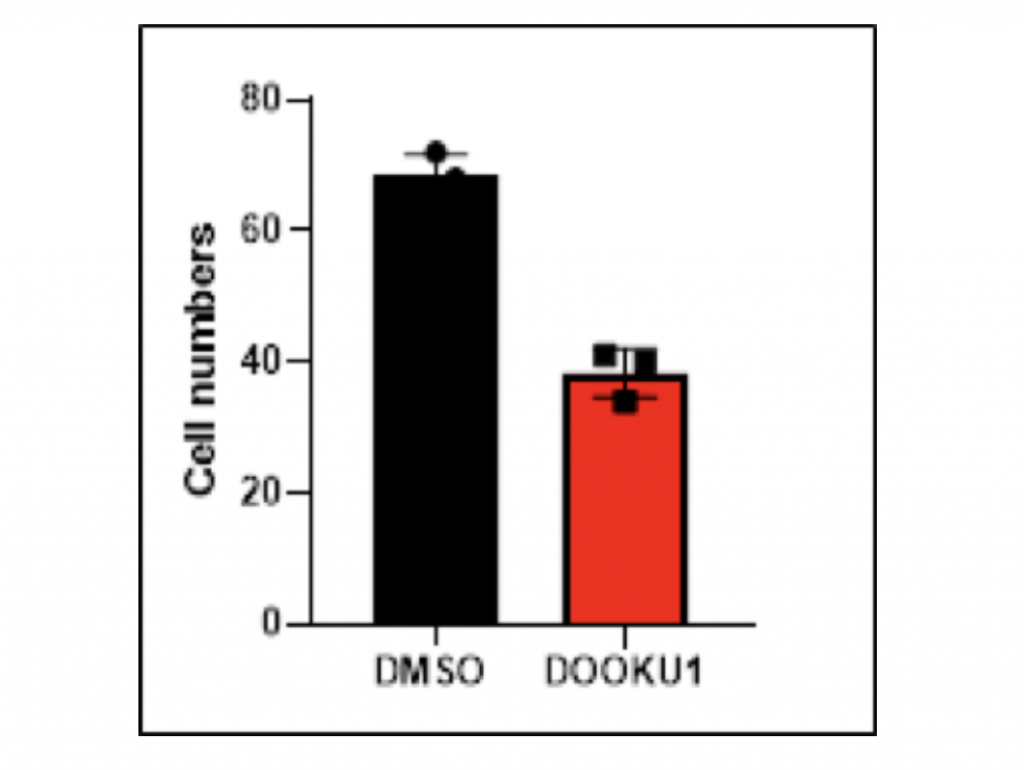
Inhibition of HCC cell migration by Dooku1 antagonist of PIEZO1/2
To examine the correlation between PIEZO1/2 and HCC metastasis besides proliferation, I again utilized the antagonist Dooku1 while culturing HCC cells in DMSO solutions. The vector group was the LM3 cells grown solely in DMSO solutions. The 48-hours migratory responses were measured by the transwell migration assay and each experiment was repeated for three times so that the average value could represent the normal case with enough data evidence. Notably, the addition of Dooku1 molecule led to decreased HCC cell migration compared to the vector. In trial 1, the amount of migrated cells with Dooku1 was 48.5% fewer than the control group, which revealed that the chemotactic ability of HCC cell metastasis was hindered by the PIEZO1/2 inhibition. Similarly for both trials 2 and 3, the quantity of transformed cells with Dooku1 was 39.7% and 44.4% fewer than the vector. In terms of the mean values, the difference between experimental Dooku1 group and control group was 44.2%. Other than quantitative data comparison, the images taken under migration assay microscope clearly manifested that the DMSO control sample contained myriads of large bulk of cells, while most of the Dooku1 group cells were small and discrete. Collectively, both qualitative and quantitative data indicate that the mechanosensitive channels PIEZO1/2 have an inhibitory relationship with the transformation ability of HCC LM3 cells.
Promotion of HCC cell metastasis by Yoda1 agonist of PIEZO1/2
The inhibitory effect of Dooku1 antagonist on HCC cell migration motivated me to conduct another experiment of whether Yoda1 could result in a stimulation of HCC transformation. Similarly, HCC LM3 cells were cultured in DMSO separately with and without Yoda1 as the experimental and control groups. Transwell migration assay was carried out to observe cells under microscope and count the quantity of successfully migrated cells after 48-hours period. Remarkably, the stimulating effect of migration was significant when Yoda1 molecules were utilized in the DMSO solution in contrast with the vector. In trial 1, the amount of migrated cells with Yoda1 was 104.2% higher than the control group. A similar trend continued for trials 2 and 3: the quantity of transformed cells with Yoda1 was 115.3% and 93.5% more than the vector. These data revealed that HCC cell metastasis was reinforced by the PIEZO1/2 activation from Yoda1 molecule. The difference between experimental group’s and control group’s average values was 104.1%. Besides experimental data, the image of Yoda1 migration was almost filled with blue-dyed cells which infers their successful transformation, while the image of the control group was comparatively scattered and scarce with cell dots. Taken together, qualitative and quantitative findings stipulate that PIEZO1/2 channels have a direct association with the ability of HCC LM3 cells to migrate.

Discussion
In the present study, I report the discovery of PIEZO1/2 as a potent mechanical regulator of growth and transformation of hepatocellular carcinoma. PIEZO1/2’s expression level is closely correlated with clinical outcomes: high level of PIEZO1/2 TPM associates with the HCC tissue while there is comparatively low level of PIEZO1/2 in adjacent tissues. Survival rates of HCC patients differ accordingly with their distinct level of PIEZO1/2 expression: there is high survival rates for HCC patients with low PIEZO1/2 TPM.
By utilizing Dooku1 to inhibit and Yoda1 to promote PIEZO1/2 activation, I ascertain the causal relationship between mechanosensitive channels and HCC development. The antagonist Dooku1 serves as an inhibitor for both proliferation and transformation of HCC LM3 cells. After 96-hours proliferation time, the growth of Dooku1 group was 54.0% fewer than the vector group, while the quantity of transformed cells with Dooku1 was averagely 44.2% fewer than the vector. On the other hand, the agonist Yoda1 acts as a stimulator for HCC growth and metastasis. After 72-hours growth period, the amount of Yoda1 group was 61.2% higher than the vector, while the average number of transformed cells with Yoda1 was averagely 104.1% more than the control group.
To further this investigation, the next step is to conduct the animal experiment instead of merely HCC LM3 cells. The host will be chosen as mice, and HCC tumors have to form in mice’s liver tissue firstly. The DMSO solution with Dooku1 and Yoda1 will be intravenous injected into mice’s blood vessels respectively to observe the size and spread of HCC tumor so that the effect of PIEZO1/2 on HCC cells can be confirmed in vivo. Moving forward, the gene for transcribing PIEZO1/2 molecules will be separately knocked out in mice so that the different impacts of PIEZO1 and PIEZO2 on HCC development can be determined. The Dooku1 and Yoda1 will be utilized, while the volume and metastasis of HCC tumor will be observed again for conclusion.
Besides my discovery, Piezo1/2 has been found by multiple studies to play a regulatory role in vascular development and progression of variety tumors. It plays a pivotal role in mediating oncogenic growth[6]. The knockdown of Piezo 1 in HCCLM3 and Hep3B notably inhibited HCC cell proliferation, migration, invasion, and epithelial–mesenchymal transition (EMT) in vitro, as well as tumor growth, metastasis, and EMT in vivo [5]. Tumor stimulant effect of Piezo1 was revealed to exert through combining Rab5c to activate TGF-β signaling pathway.
In summary, by using bioinformatic analysis, cell counting kit-8, and transwell migration assay, I have identified PIEZO1/2 as a tumor regulating mechanical-force channel. Any inhibition or promotion of PIEZO1/2 expression level in tissues could result in enhancement or hindrance of HCC proliferation and metastasis. These findings shed new light on a novel prognostic biomarker and a potential therapeutic target in HCC for diagnosis. Plus, Dooku1 molecules can be constituents of drugs design for new therapeutic treatments.
Materials and Methods
a. TranswellMigration:
This assay measures the chemotactic capability of cells toward a chemo-attractant, which allows scientists to quantify cell movement. The assay is based on two compartments separated by a membrane with an accurately defined pore size. A potential chemotactic factor is placed in one compartment and a gradient develops across the membrane. Hepatocellular carcinoma cells placed in the other compartment migrate along with this gradient. Within several hours after adding the test substance, the number of cells that have migrated through the membrane can be counted.
b. Cell culture:
Hepatocellular carcinoma cells were cultured in DMEM supplemented with 1-% FBS, 2mM-glutamine, and penicillin/streptomycin at 37°C under 5% CO2 in a humidified chamber
c. CellcountingKit-8assay:
It allows sensitive colorimetric assays for the determination of cell viability in cell proliferation and cytotoxicity assays. As alive cells number increase, the formazan formed increases as well leading to a darker color
d. Wound Healing:
An assay performed completely in vitro situation to detect the directional cell migration, migration rate and mimic cell migration that occurs in vivo. Firstly, produce a single layer of cells called monolayer. Then create a wound in the cell plate just scraping off some cells from the surface. Once the wound is created, the migration of cells can be studied, since the old cell will start to divide and fill the surface as they won’t allow any gap in surface area. After creating the wound, take a microscopic picture and then after every single time interval when the cell starts to migrate, take pictures to see what is the directionality of news cell to migrate. By comparing images one after another from the beginning of the wound to the healing of the wound, migration rate can also be calculated.
References
[1]Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin.
[2]Yang, J.D., Hainaut, P., Gores, G.J. et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16, 589–604 (2019). https://doi.org/10.1038/s41575-019-0186-y
[3]Jiang, Y., Zhang, H., Wang, J. et al. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol 15, 34 (2022). https://doi.org/10.1186/s13045-022-01252-0
[4]“Nobel Prizes 2021.” NobelPrize.Org, www.nobelprize.org/prizes/medicine/2021 advanced-information. Accessed 11 June 2022.
[5]Li, Ym., Xu, C., Sun, B. et al. Piezo1 promoted hepatocellular carcinoma progression and EMT through activating TGF-β signaling by recruiting Rab5c. Cancer Cell Int 22, 162 (2022). https://doi.org/10.1186/s12935-022-02574-2
[6]Yu, Jia-Lin, and Hai-Yang Liao. “Piezo-Type Mechanosensitive Ion Channel Component 1 (piezo1) in Human Cancer.” Biomedicine & Pharmacotherapy, Elsevier Masson, 16 May 2021, www.sciencedirect.com/science/article/pii/S0753332221004741#!
About the author

Yicheng Ding
Yicheng is a senior at the Shanghai Pinghe Bilingual School. He is strongly interested in biology, especially molecular biology related to human bodies, such as proteins, DNA, and tumors. In his spare time, Yicheng usually plays table tennis and basketball with friends to strengthen physical strength.